Precision Medicine and Biologics
Latest News
Latest Videos
CME Content
More News
The company is set to meet with the FDA in August to discuss the epidermolysis bullosa drug’s anticipated BLA.

Coverage for eligible patients began July 12, 2023.

The inaugural ACBC Texas Round Up 2023 meeting kicks off this week to further promote biologic access training.

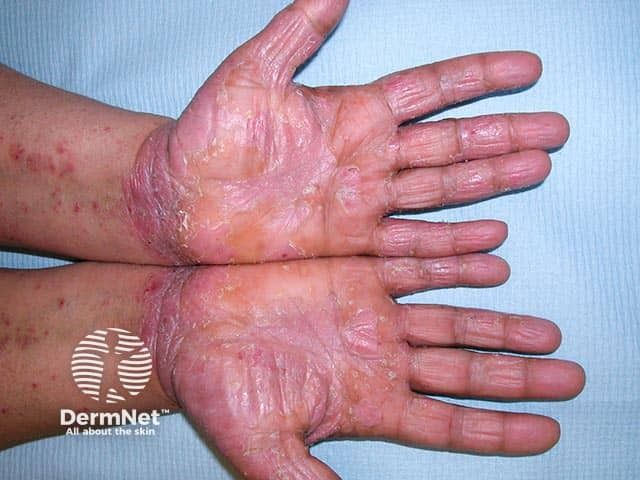
Highly effective medications for severe disease are expensive and administered invasively, while the development of treatments for milder disease has lagged, a new review on psoriasis clinical trials concludes.

Precision medicine may help improve the diagnosis and management of melanoma.

A new study highlights the ways genetic testing and profiling can be extended to help diagnose and treat a variety of conditions—even rashes.
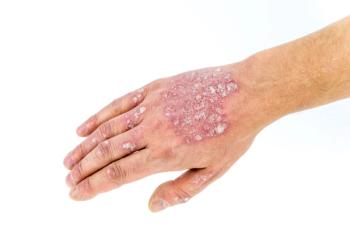
The amount of transcriptomic data available for psoriasis may prove helpful in treating the condition through a precision medicine approach.

Studies show that patients with atopic dermatitis may benefit from a multidisciplinary approach.

Precision medicine offers better results while treating severe atopic dermatitis.
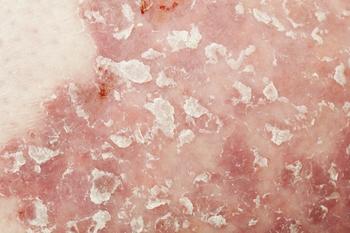
Minimally invasive method outperformed tape stripping in a recent study.

The most critical step is understanding what value precision medicine brings to your practice, and where it can have greatest impact from both a clinical and business perspective.

A recent study dove into how precision medicine can be used to better target inflammatory skin disease and provide physicians with better treatment regimens.
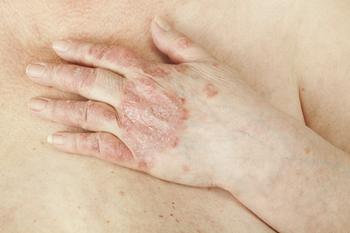
Mindera Health and a national health insurance company launched a pilot program to improve moderate-to-severe psoriasis management with precision medicine.

Recent data tracks the first realization of precision medicine for patients with psoriasis.

Click here to answer this weeks poll.

In 2023 as many as 11 biosimilars to Humira are headed for the US market.

A phase 3 study conducted by Amgen found that the company’s ustekinumab biosimilar was as safe and efficacious as the reference product (Stelara) in patients with moderate to severe plaque psoriasis.

Experts explored out-of-the-box solutions for treating challenging skin and hair disorders at the American Academy of Dermatology (AAD)’s 2022 Annual Meeting held March 25 to 29, 2022, in Boston, Massachusetts.

If approved, dupilumab will be the first biologic medicine available in the US to treat uncontrolled moderate to severe atopic dermatitis for young children 6 months to 5 years old.

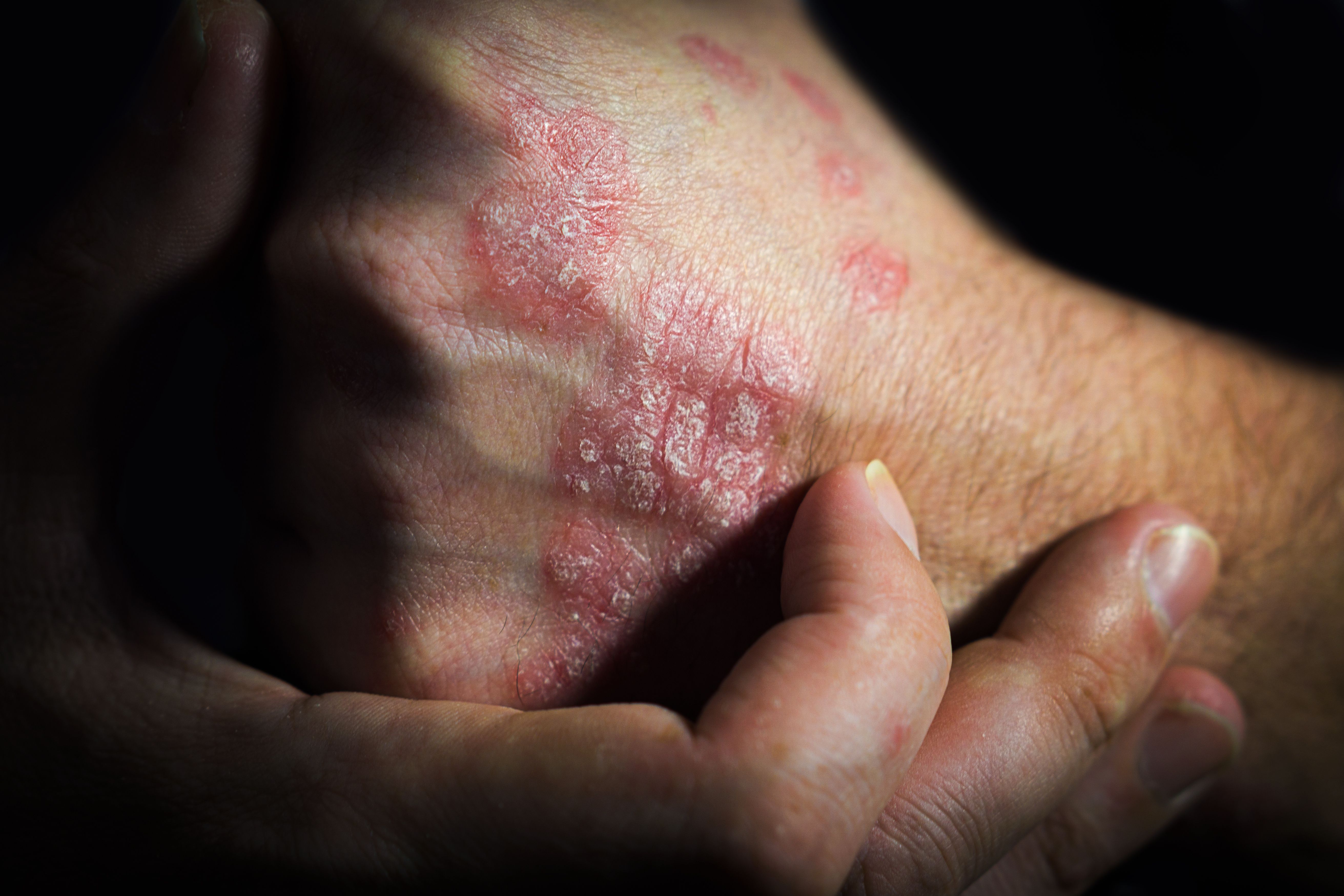
Studies offer positive outcomes for patients with conditions like hidradenitis suppurativa or psoriasis.

Secukinumab is the only biologic treatment in the US that’s approved by the FDA for pediatric patients with enthesitis-related arthritis (ERA) and psoriatic arthritis (PsA).

An article published in the Journal of Investigative Dermatology examined genetic, environmental triggers, immunology, pathophysiology, and precision medicine in psoriasis.

This episode highlights findings from an investigative study exploring the molecular targeting of biologics for psoriasis, the IL-17 drug class, how bimekizumab is different, plus more.

Taking the polar opposite of a one-size-fits-all approach, clinicians now factor in a patient’s genetic profile, medical history, microbiome composition, lifestyle, and diet, among other parameters, otherwise known as precision medicine.