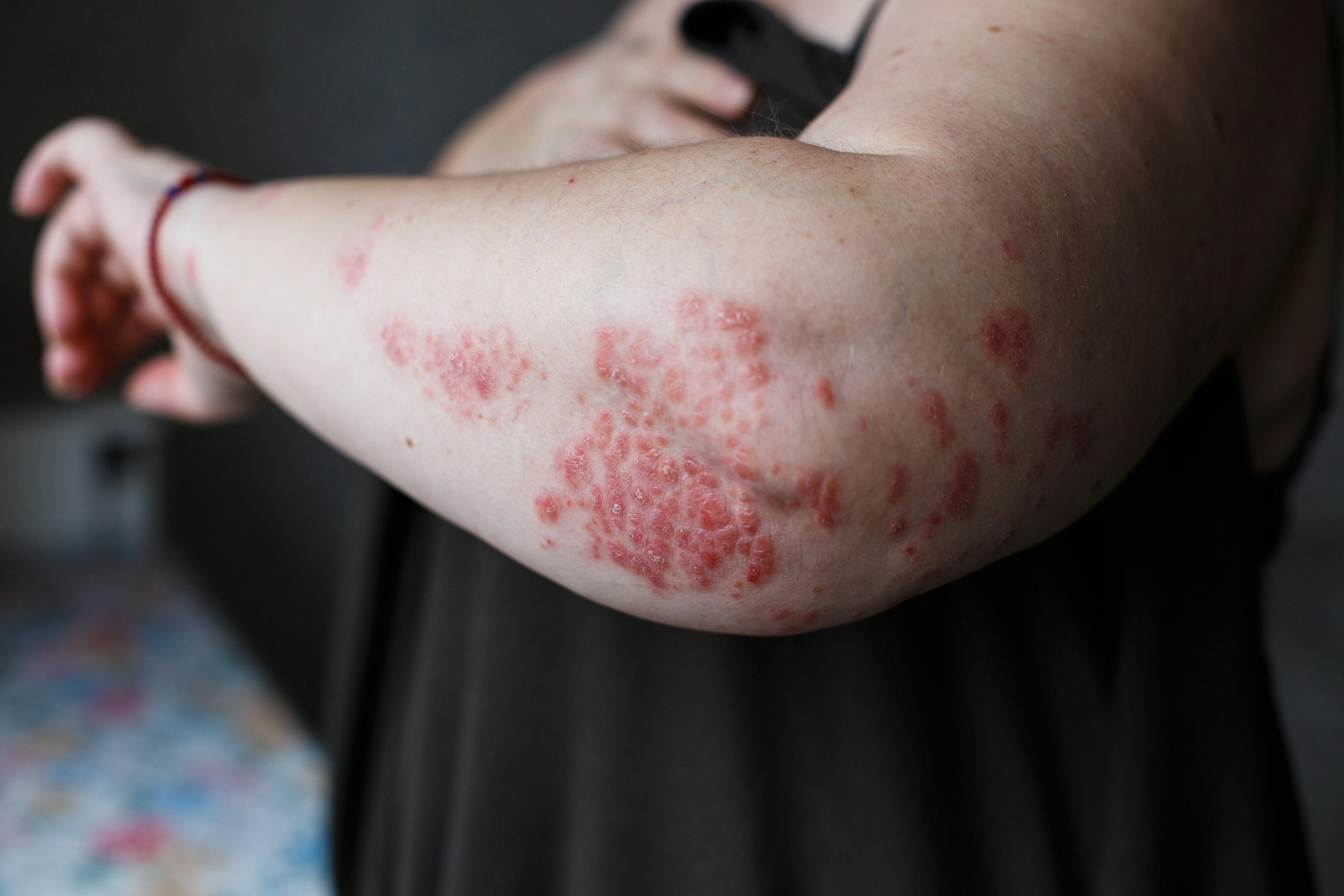
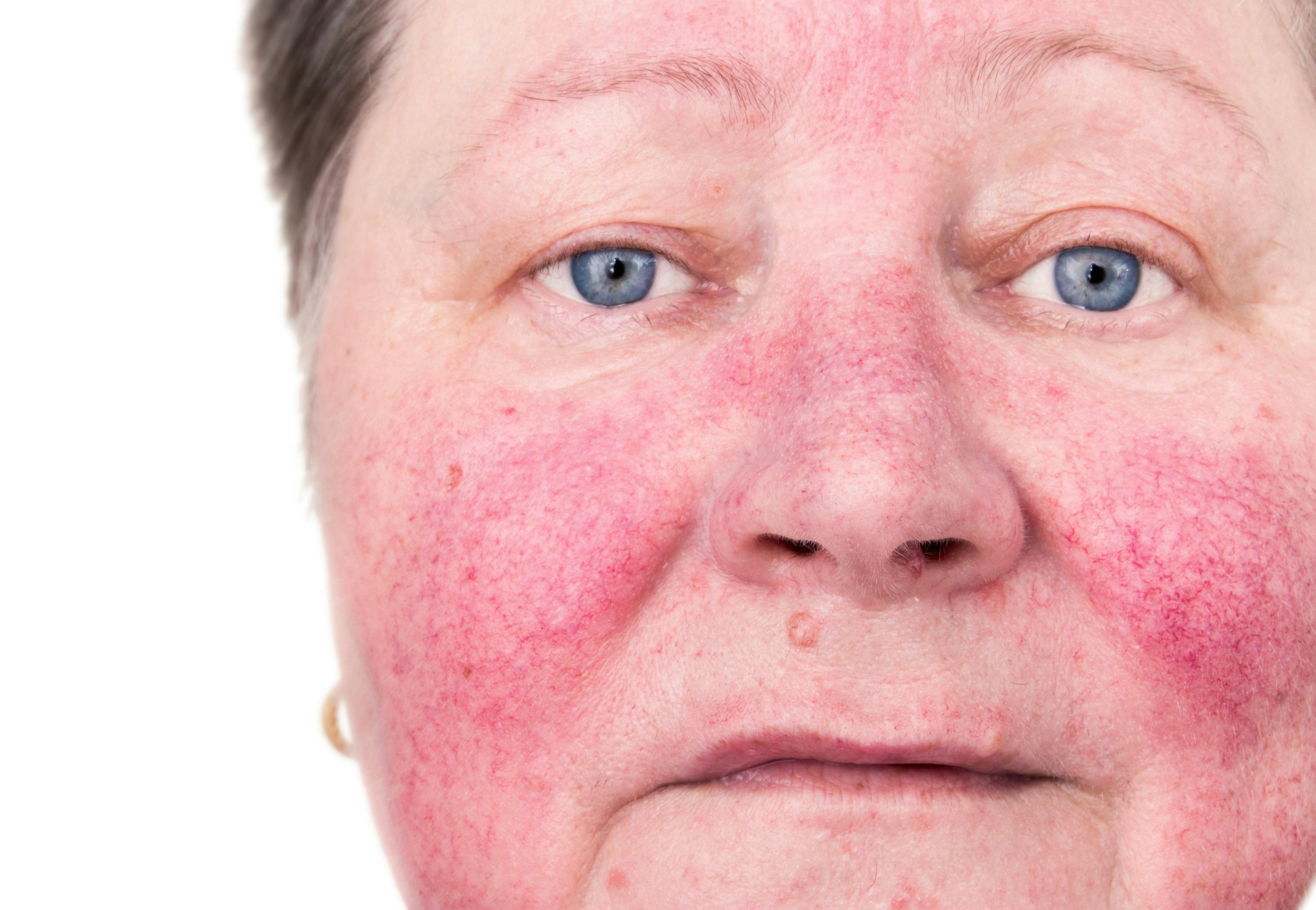
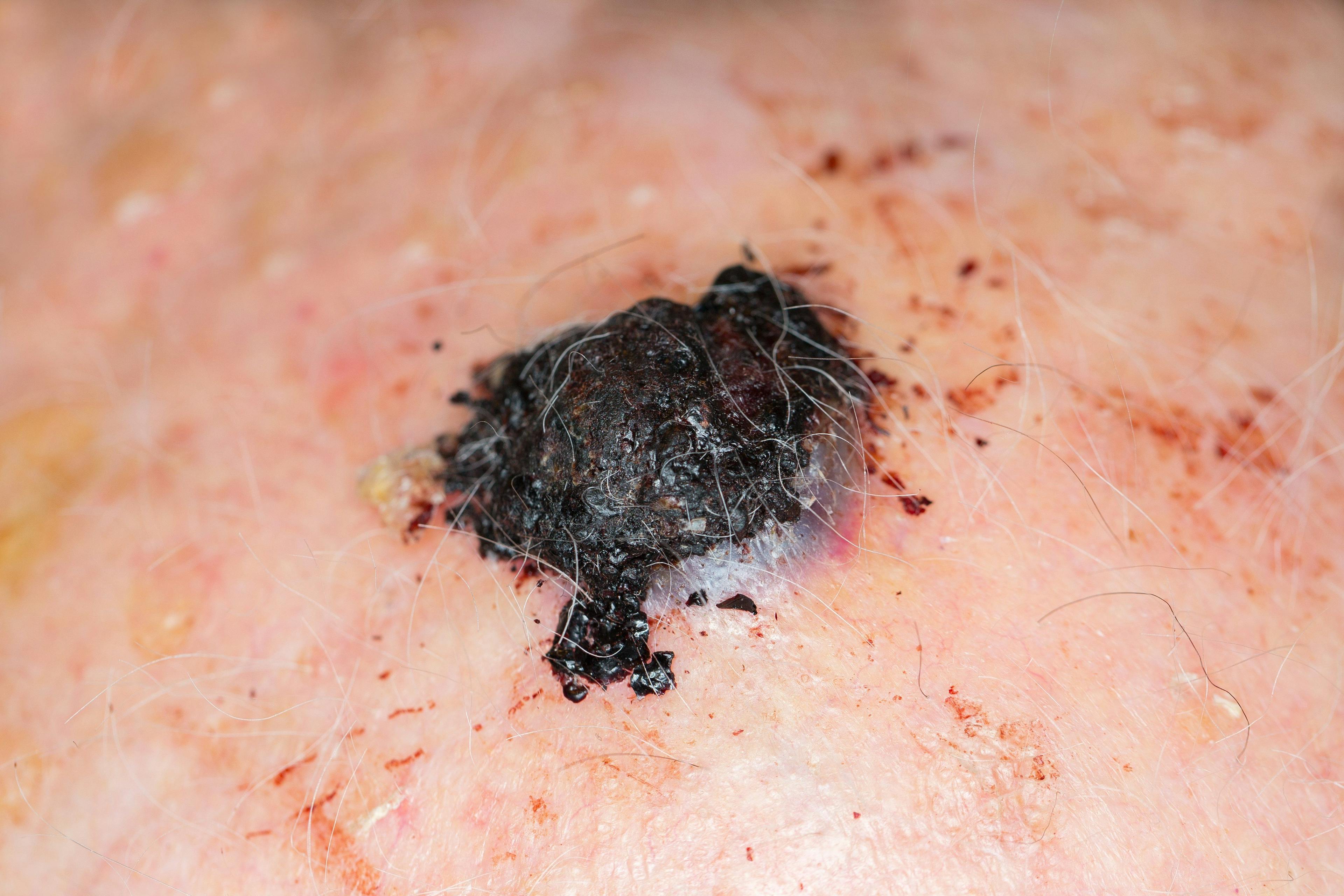
- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Atopic dermatitis pipeline full of potential
Author(s):
Atopic dermatitis therapy is currently undergoing a revolution that promises to treat patients of all ages who are suffering from every aspect of the disease-from rashes, itch and sleep disturbances, to secondary effects, like anxiety and depression.
Dr. Eichenfield

As dermatologists gain experience with newer atopic dermatitis medications, they’re getting glimpses of data from a pipeline full of potential therapies, according to Lawrence Eichenfield, M.D.
RELATED: Abrocitinib works quickly in atopic dermatitis
It’s a revolution in atopic dermatitis therapy that promises to treat patients of all ages and suffering from every aspect of the disease-from rashes, itch and sleep disturbances, to secondary effects, like anxiety and depression, he says.
EXPANDING INDICATIONS
Researchers are studying approved atopic dermatitis drugs in younger patients, according to Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital San Diego, and professor of dermatology and pediatrics at the University of California San Diego School of Medicine. Pfizer recently announced that it completed a study of topical crisaborole (Eucrisa) in atopic dermatitis patients younger than two years.
“It appeared safe, but we haven’t yet heard the details of the data and the status of expanded specific indication for use of crisaborole under age two,” says Dr. Eichenfield, who also is on the National Eczema Association’s Scientific Advisory Committee.
The FDA has approved dupilumab (Dupixent, Sanofi and Regeneron Pharmaceuticals), an interleukin (IL)-4 and IL-13 blocker, in adults and patients ages 12 to 17 years with moderate-to-severe atopic dermatitis.
“Studies have been completed down to six years of age, and they’re finishing studies down to under two years,” Dr. Eichenfield says. “The community is well aware that we have children across the ages that have very severe disease. Historically, there have been no systemic drugs, other than corticosteroids, approved for pediatric and adolescent atopic dermatitis until the approval of dupilumab age 12 and older.”
RELATED: Oral JAK inhibitor appears safe, effective for atopic dermatitis
PIPELINE OF POTENTIAL
Dr. Eichenfield points to an unprecedented array and steady stream of newer topical and systemic agents that are in various phases of drug development.
“We continue to see portions of the data as they move along from phase 2 to phase 3 trials,” he says.
There are two IL-13 inhibitors well under development: tralokinumab (Leo Pharma) and lebrikizumab (Dermira).
“This brings forward the intriguing question of whether they’re going to be as e ective or less e ective than IL-4 and IL-13 blocker [like dupilumab]. They may be more selective. There are di erences in how the drugs work in terms of how they block IL-13 signaling. We’re awaiting those studies,” Dr. Eichenfield says.
Yet another biologic target in atopic dermatitis is IL-31. IL-31 blocker nemolizumab (Galderma) has been in clinical studies.
“We’re waiting for further results to see how that could potentially be a niche therapy for moderate-to-severe disease,” Dr. Eichenfield says.
RELATED: Can sweat shed a light on disease severity in atopic dermatitis?
Immune targeted therapies in the pipeline include GBR830, an OX40 immunoglobulin G antibody. This immune checkpoint receptor appears to be important in T-cell activity, according to Dr. Eichenfield. There are two companies developing OX40 inhibitors for atopic dermatitis: Kyowa Hakko Kirin and Glenmark Pharmaceuticals.
“This will be a relatively specific immune target but whether it has broader immunosuppression is unknown until we see the clinical trial results,” according to Dr. Eichenfield.
There are several Janus kinase (JAK) inhibitors by many drug companies being studied for atopic dermatitis. JAK inhibitors are small molecule medications-more traditional type oral drugs that are not antibody based. The JAK-signal transducer and activator of transcription (STAT) pathway appears important in a variety of inflammatory diseases, including atopic dermatitis. This pathway is also being studied for alopecia areata and vitiligo treatment, according to Dr. Eichenfield.
The three JAK inhibitor drugs that are furthest along in development for atopic dermatitis are Eli Lilly and Company drug baricitinib; Abbvie’s upadacitinib; and Pfizer’s abrocitinib.
“They have selectivity to different JAK pathways as well as differences in other aspects of drug metabolism. Those differences may influence their effiacy and side effect profiles,” Dr. Eichenfield says. “We’re aware that some of the JAK inhibitors used to treat other diseases, such as tofacitinib (Xeljanz, Pfizer), have received safety warnings because of systemic side effects including venous thrombosis. There are potential effects that may require standard blood work monitoring. As we go forward assessing these drugs, we’ll be assessing safety.”
RELATED: Atopic dermatitis treatment hurdles
Interestingly, companies are developing topical JAK inhibitors. Some think topical formulations might minimize the chance of systemic side effects, Dr. Eichenfield says.
“The furthest along that we’ve seen phase 2 and phase 3 trials are for topical ruxolitinib (Incyte) and topical delgocitinib (Leo Pharma),” he says.
In other topical news, DS Biopharma has developed the investigational topical lipid-based product 5% DS107 cream to use along with topical corticosteroids to maintain results from topical corticosteroids and potentially discontinuing their use.
Tapinarof cream (Dermavant), a topical aryl hydrocarbon receptor modulating agent (TAMA) topical, is a novel treatment being developed for psoriasis and atopic dermatitis, according to Dr. Eichenfield.
“There are other drugs that are in earlier stages of development. It’s an interesting diverse landscape for medications,” he says. “We know that we need more drugs to treat atopic dermatitis, as we have increasing evidence of the impact of moderate-to-severe disease and its association with high rates of comorbidities and other health effects, including ADHD [attention deficit hyperactivity disorder], anxiety, depression and more. We also know that many patients have been undertreated due to concerns about topical steroids and corticosteroid phobia. With the introduction of new topical and systemic therapies, we have the promise that we can minimize the disease’s impact on individuals.”
References:
Dr. Eichenfield has consulting and/or research ties to Allergan, Almirall, Dermira, DS Laboratories, Galderma, Glenmark, Incyte, LEO Pharma, Lilly, Novan, Otsuka Pharmaceutical Co., Pfizer, Sanofi-Regeneron, Ortho Dermatologics/Valeant.