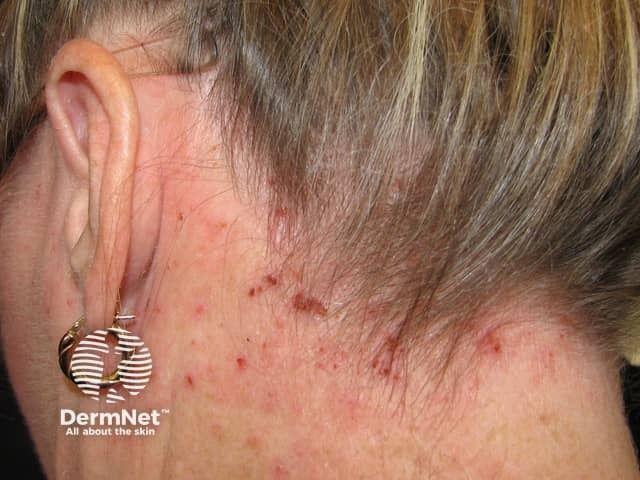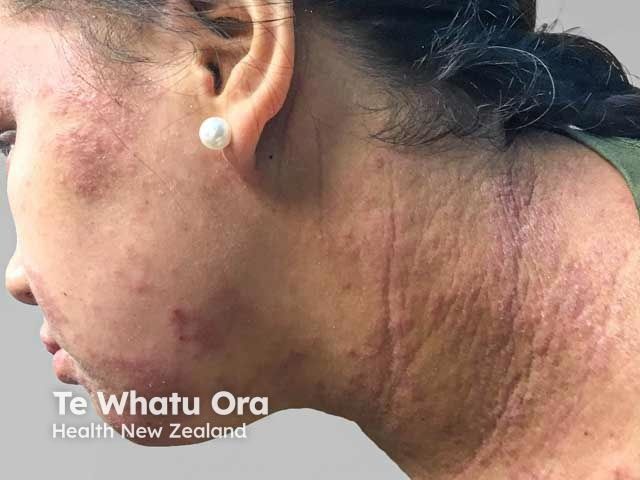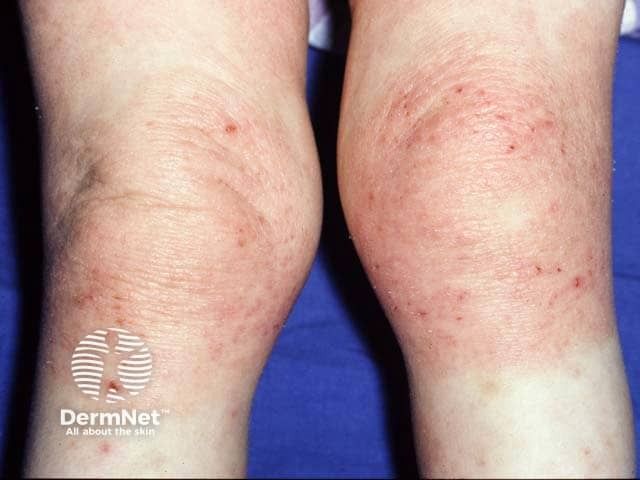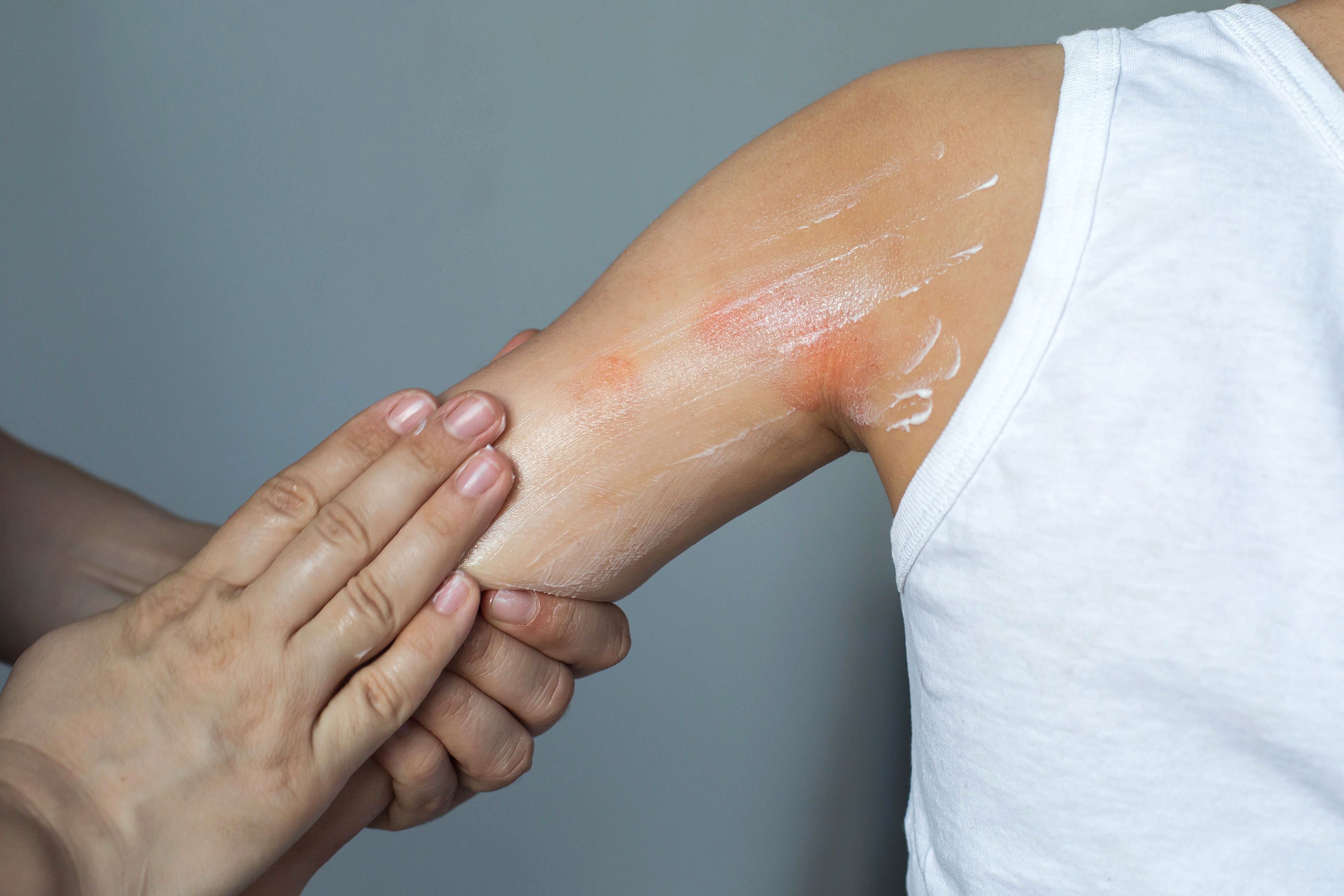
Atopic Dermatitis
Latest News
Video Series

Latest Videos
Shorts
CME Content
More News

Phase 1 cohort 4 data show soquelitinib demonstrated sustained clinical activity through 8 weeks, including in patients with prior systemic therapy exposure.

A Winter Clinical Hawaii poster determined that roflumilast cream 0.05% is well tolerated and effective for diverse patient populations with atopic dermatitis

Connect Biopharma reveals innovative data on rademikibart's mechanism of action, highlighting its potential impact on atopic dermatitis and future clinical priorities.
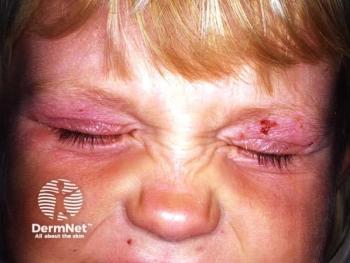
Dupilumab demonstrates significant efficacy and safety in treating severe atopic dermatitis in children aged 6 months to 5 years, improving quality of life.
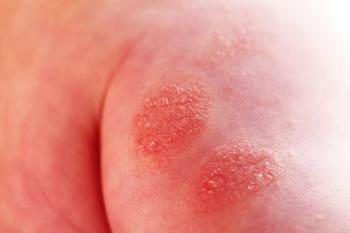
Johnson & Johnson reveals JNJ-95475939's early termination due to insufficient efficacy in treating moderate to severe atopic dermatitis.
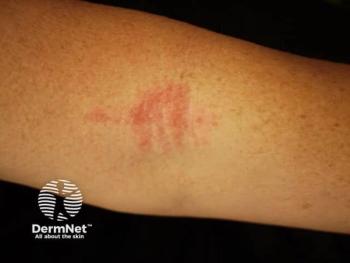
Apogee Therapeutics reveals promising phase 1b trial results for zumilokibart, highlighting its potential as a durable treatment for atopic dermatitis.
Expert consensus clarifies systemic corticosteroid use in atopic dermatitis, emphasizing risks and advocating for advanced therapies for better patient outcomes.

Discover the 2025 advancements in AD treatment, including new therapies, pediatric approvals, and insights into effective management strategies.

Brad Glick, DO, MPH, and attendees discuss innovative treatments for atopic dermatitis, emphasizing the importance of patient comfort and modern nonsteroidal options.

At a recent Case-Based Roundtable® event, Aaron Farberg, MD, moderated a discussion of challenging atopic dermatitis cases spanning adult, adolescent, and pediatric patients.

Early-phase development introduces a potential first-in-class oral approach targeting type 2 inflammation relevant to dermatologic diseases.
Discover the latest breakthroughs in atopic dermatitis and psoriasis treatments for 2025, enhancing patient care and individualizing therapy options.
Wildfire air pollution significantly increases atopic dermatitis and itch-related dermatologic visits, highlighting the urgent need for skin protection during poor air quality.
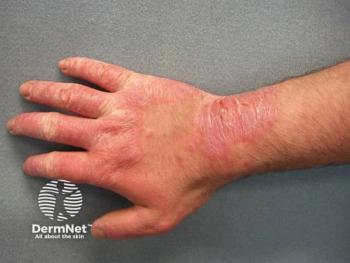
Comprehensive patch testing is vital for accurately diagnosing various forms of dermatitis, improving patient care, and addressing public health challenges.
Kymera Therapeutics reveals promising phase 1b results for KT-621, an oral STAT6 degrader, showing significant effects in moderate to severe atopic dermatitis.

Experts explore the integration of AI in atopic dermatitis management, highlighting benefits, risks, and the need for cautious implementation in patient care.

At the Horizons in Advanced Practice meeting, Omar Noor, MD, led NP and PA attendees through 3 complex cases of atopic dermatitis, prurigo nodularis, and chronic spontaneous urticaria.

Lynk Pharmaceuticals reveals promising phase 2 trial results for LNK01004, a topical treatment showing efficacy and safety in moderate-to-severe atopic dermatitis.

LEO Pharma reveals promising 32-week results for tralokinumab in treating atopic dermatitis on hands, enhancing patient quality of life and safety.
Robert Bissonnette, MD, presented tape-strip transcriptomics detecting meaningful steroid responses after just 1 day of therapy.

Arcutis advances pediatric atopic dermatitis treatment with roflumilast cream, completing enrollment in key INTEGUMENT-INFANT trial.

The exclusive event brought together physician assistants and nurse practitioners from across the country to review complex atopic dermatitis, psoriasis, chronic hand eczema, and hidradenitis suppurativa patient cases.

Findings suggest immune dysregulation and chronic inflammation in atopic dermatitis may contribute to increased skin cancer susceptibility.

Skin pain in atopic dermatitis significantly impacts quality of life, necessitating its recognition and treatment as a vital symptom for better patient outcomes.

Castle Biosciences’ new test uses molecular signatures from lesional skin to guide systemic therapy decisions in moderate to severe AD.