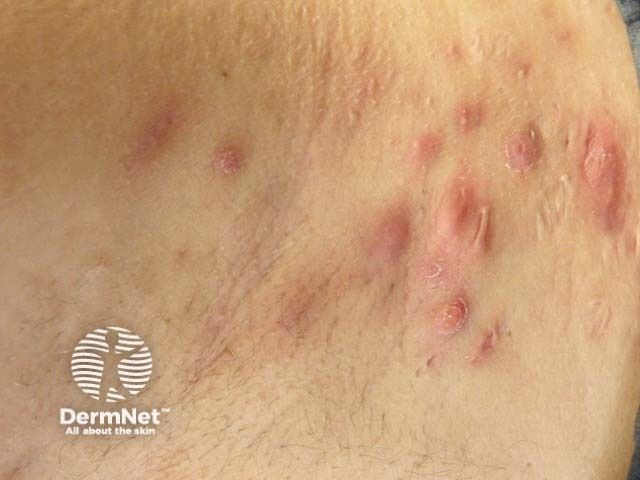
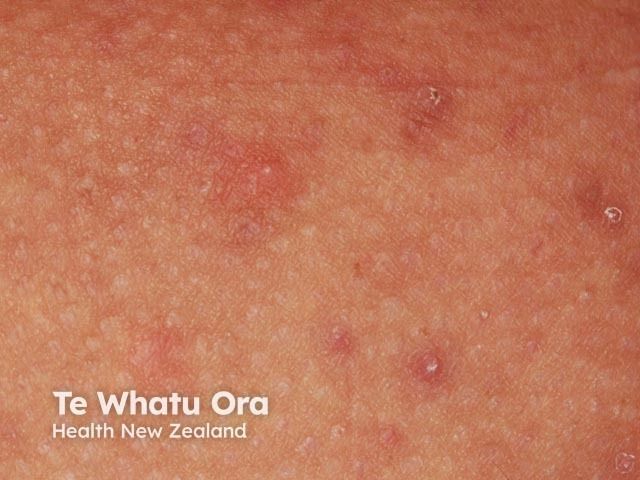
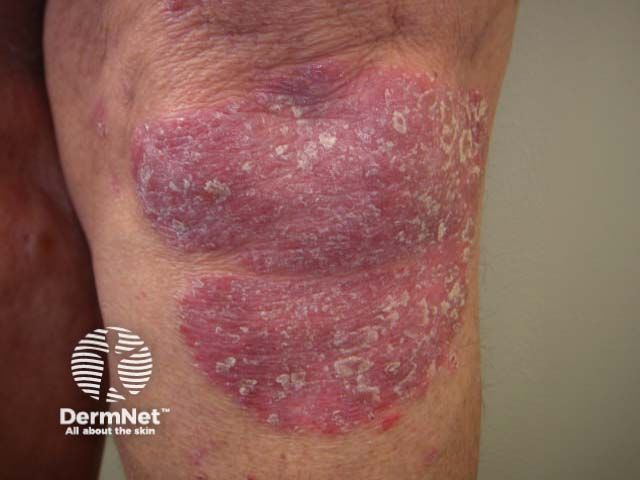
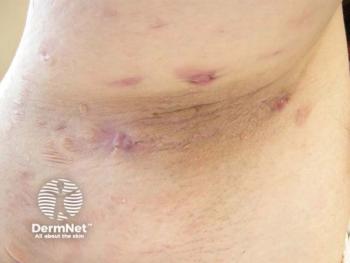
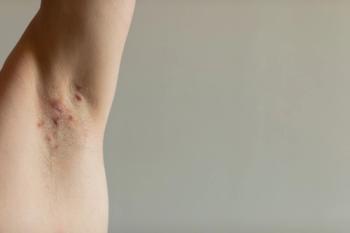
Hidradenitis Suppurativa
Latest News
Latest Videos

CME Content
More News
Navigator Medicines advances NAV-240 and NAV-242, targeting dual inflammation pathways for hidradenitis suppurativa in upcoming clinical trials.

MoonLake Immunotherapeutics shares promising updates on sonelokimab's efficacy for hidradenitis suppurativa.

At the Dermatology Times Horizons in Advanced Practice meeting in Tampa, Florida, conference chair Lakshi Aldredge, MSN, ANP-BC, DCNP, focused a discussion on efficacy data for adalimumab, secukinumab, and bimekizumab.
Discover the latest advancements in hidradenitis suppurativa therapies, including promising biologics and oral treatments reshaping patient care in 2025.

At a recent Case-Based Roundtable event, Harrison Nguyen, MD, MBA, MPH, reviewed 2 HS patient cases and discussed the role of biologics.

Porter leads a dynamic roundtable on hidradenitis suppurativa, exploring innovative treatments and collaborative strategies for improved patient outcomes.

Put your dermatology expertise to the test with Dermatology Times’ interactive quiz series!
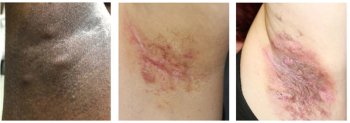
A new global study reveals hidradenitis suppurativa affects 1 in every 100 people, highlighting the need for increased awareness and improved health care strategies.

At a Dermatology Times Case-Based Roundtable event, Joe Gorelick, MSN, FNP-C, reviewed real-world strategies for hidradenitis suppurativa, highlighting growing preference for bimekizumab based on its dual IL-17A/IL-17F inhibition.

Navigator Medicines advances NAV-240, a promising bispecific antibody for HS, targeting dual pathways for enhanced treatment efficacy.

Participants agreed that HS is rarely controlled with monotherapy and often requires coordinated multimodal care.

Discover the potential of INF904, a groundbreaking oral therapy targeting C5aR for hidradenitis suppurativa and chronic spontaneous urticaria, promising new treatment options.
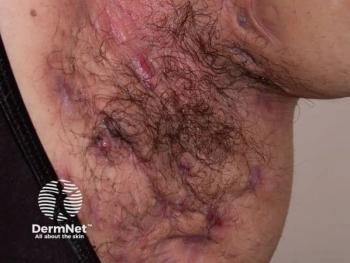
Discover the latest insights on hidradenitis suppurativa treatment, emerging therapies, and the importance of compassionate care for patients.

Karan Lal, DO, explores the transformative role of GLP-1 therapies in dermatology, highlighting their potential in treating inflammatory skin diseases and enhancing aesthetic care.

The topline data support advancing INF904 into larger, controlled trials as a potential convenient alternative to injectable biologics.

At the 10th Annual Symposium on Hidradenitis Suppurativa Advances, Ralph George, MD, FRCS, emphasized that the growing collaboration between medical and surgical management is redefining the role of procedures in HS.

Incyte launches Ingenuity Awards to empower HS community, funding innovative patient-driven initiatives for better treatment and outcomes in hidradenitis suppurativa.

UCB reveals three-year data on bimekizumab, showcasing significant pain relief and lesion resolution in hidradenitis suppurativa patients at the 2025 SHSA.

Avalo Therapeutics completes enrollment in the LOTUS trial for AVTX-009, targeting hidradenitis suppurativa with promising IL-1β inhibition.

According to a poster at Fall Clinical 2025, over 80% of patients remained flare-free across the 2-year period.
James Del Rosso, DO; April Armstrong, MD, MPH; Mark Nestor, MD, PhD; Mark Lebwohl, MD; Linda Stein Gold, MD; and Darrell Rigel, MD, MS, presented some of the biggest updates in dermatology to close out the year.

CO₂ laser treatment significantly enhances quality of life for hidradenitis suppurativa patients, showing promising outcomes in a large prospective study.
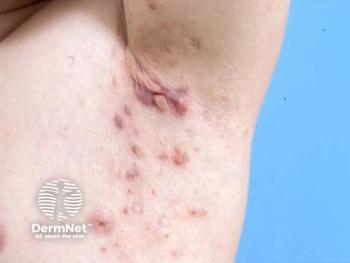
Emerging biologic combinations may open a new therapeutic frontier for severe hidradenitis suppurativa (HS) that fails to respond to conventional and single-pathway biologics.

This review of the latest dermatologic studies highlights new research on hidradenitis suppurativa, including associated pediatric comorbidities, CO2 laser treatment, impact on sexual health, and more.
While VELA-1 achieved statistical significance under all analyses, VELA-2 narrowly missed its composite primary endpoint due to higher-than-expected placebo outcomes.