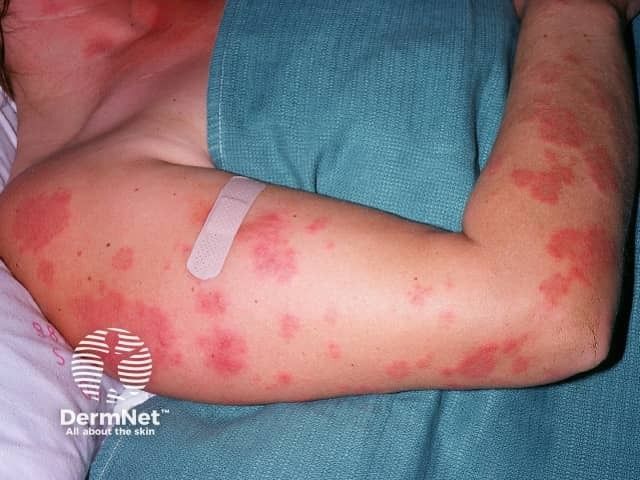
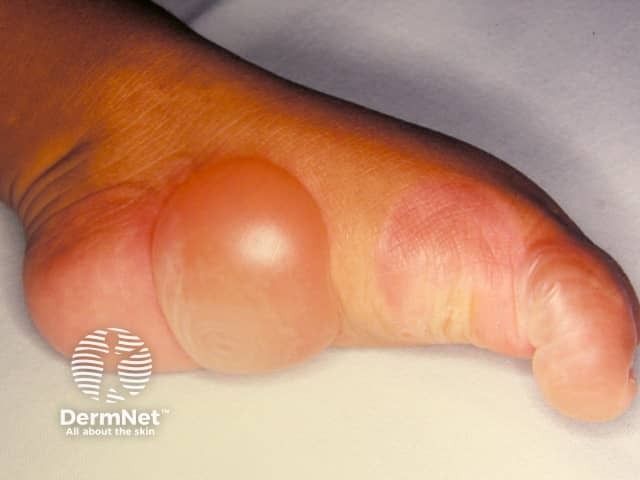
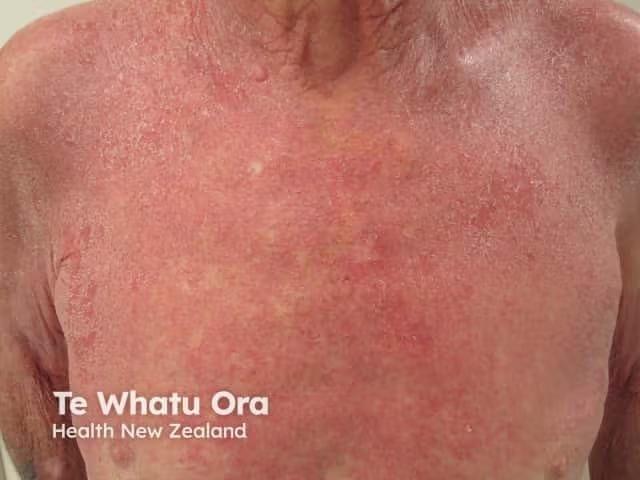
Rare Disease
Latest News
Latest Videos
Shorts

CME Content
More News
Karen McGuire, PhD, discusses innovative TolaSure for treating epidermolysis bullosa simplex, focusing on disease modification rather than symptom management.
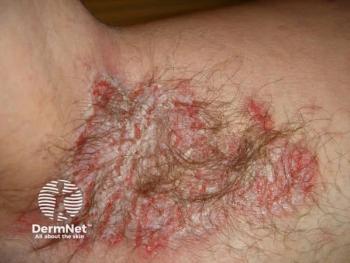
Researchers explore CO₂ laser therapy's effectiveness for refractory HHD, revealing its potential to restore skin health and reduce inflammation.

The first treatment follows Zevaskyn’s FDA approval as the first autologous, gene-modified cellular sheet designed to address RDEB wounds.

Quoin Pharmaceuticals' QRX003 granted FDA Orphan Drug Designation, advancing treatment for Netherton Syndrome, a rare dermatological disease.
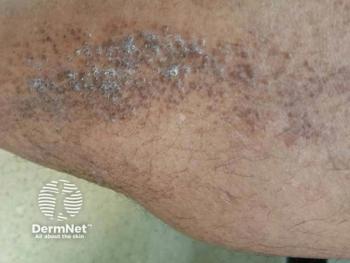
Selective JAK1 inhibition with upadacitinib may offer a fast-acting, steroid-sparing option for refractory lichen amyloidosis by interrupting IL-31–mediated itch and the itch–scratch cycle.
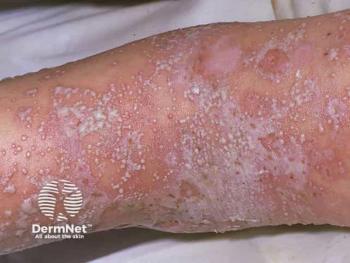
Spesolimab joins LEO Pharma’s dermatology portfolio to further expand spesolimab’s reach for patients worldwide with generalized pustular psoriasis.

Disc Medicine filed an NDA for bitopertin to treat erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP) in patients ≥12 years.

Krystal Biotech enhances B-VEC's label, empowering DEB patients with at-home treatment options and improving quality of life through innovative gene therapy.
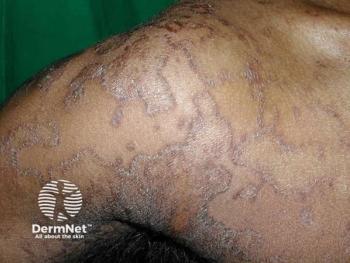

A case report presents a unique case of Bowen's disease affecting the lower extremities in a 75-year-old woman, which was initially misdiagnosed as a fungal infection.

Karen McGuire, PhD, discusses TAMES-02, a clinical trial evaluating TolaSure for Epidermolysis Bullosa Simplex.


Orphan designation provides Soligenix with potential regulatory, financial, and exclusivity benefits as it develops SGX945.

Shannon C. Trotter, DO, FAOCD, FAAD, and patient Janene Tirado, highlight GPP Awareness Day and the urgent need for recognition and understanding of generalized pustular psoriasis.
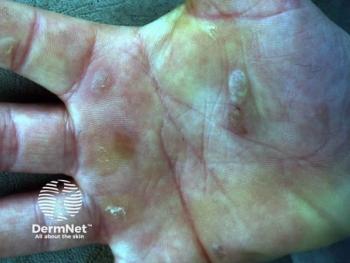
BioMendics initiates TAMES-02 trial for TolaSure, a groundbreaking therapy targeting the root cause of Epidermolysis Bullosa Simplex, offering new hope.
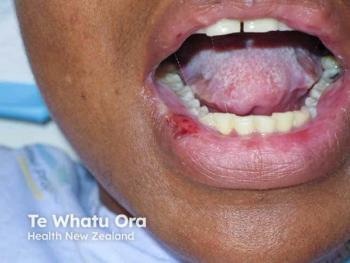
The open-label trial showed dusquetide produced similar results to apremilast in reducing oral ulcers, pain, and ulcer duration.

PC111, a novel therapy from Scinai, targets pemphigus vulgaris and SJS/TEN, offering rapid relief without immunosuppression.

Quoin Pharmaceuticals advances QRX003 for Netherton Syndrome, receiving FDA's RPD Designation and showing promising clinical results.

Galderma launches clinical trials for nemolizumab, targeting systemic sclerosis and chronic pruritus of unknown origin, addressing critical patient needs.

Abeona Therapeutics' VIITAL trial reveals promising results for RDEB treatment, showcasing significant wound healing and pain relief with Zevaskyn therapy.

A new partnership aims to revolutionize topical treatments for epidermolysis bullosa, enhancing patient care with innovative drug delivery technology.

Quoin Pharmaceuticals advances QRX003 for Netherton Syndrome, receiving FDA clearance for a pivotal trial to assess its effectiveness and safety.

ZEVASKYN, the first FDA-approved gene therapy for wounds in recessive dystrophic epidermolysis bullosa, is now available at Lurie Children's Hospital.

A pediatric patient with Peeling Skin Syndrome observed overall skin healing with no adverse events after 12 weeks of treatment.

The FDA approved pz-cel, a groundbreaking gene therapy for recessive dystrophic epidermolysis bullosa, transforming treatment options for patients.