- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Potential first-line treatment for advanced cutaneous squamous cell carcinoma
Author(s):
The epidermal growth factor (EGFR) inhibitor cetuximab achieved good outcomes in patients with advanced SCC, according to results of a recent study.
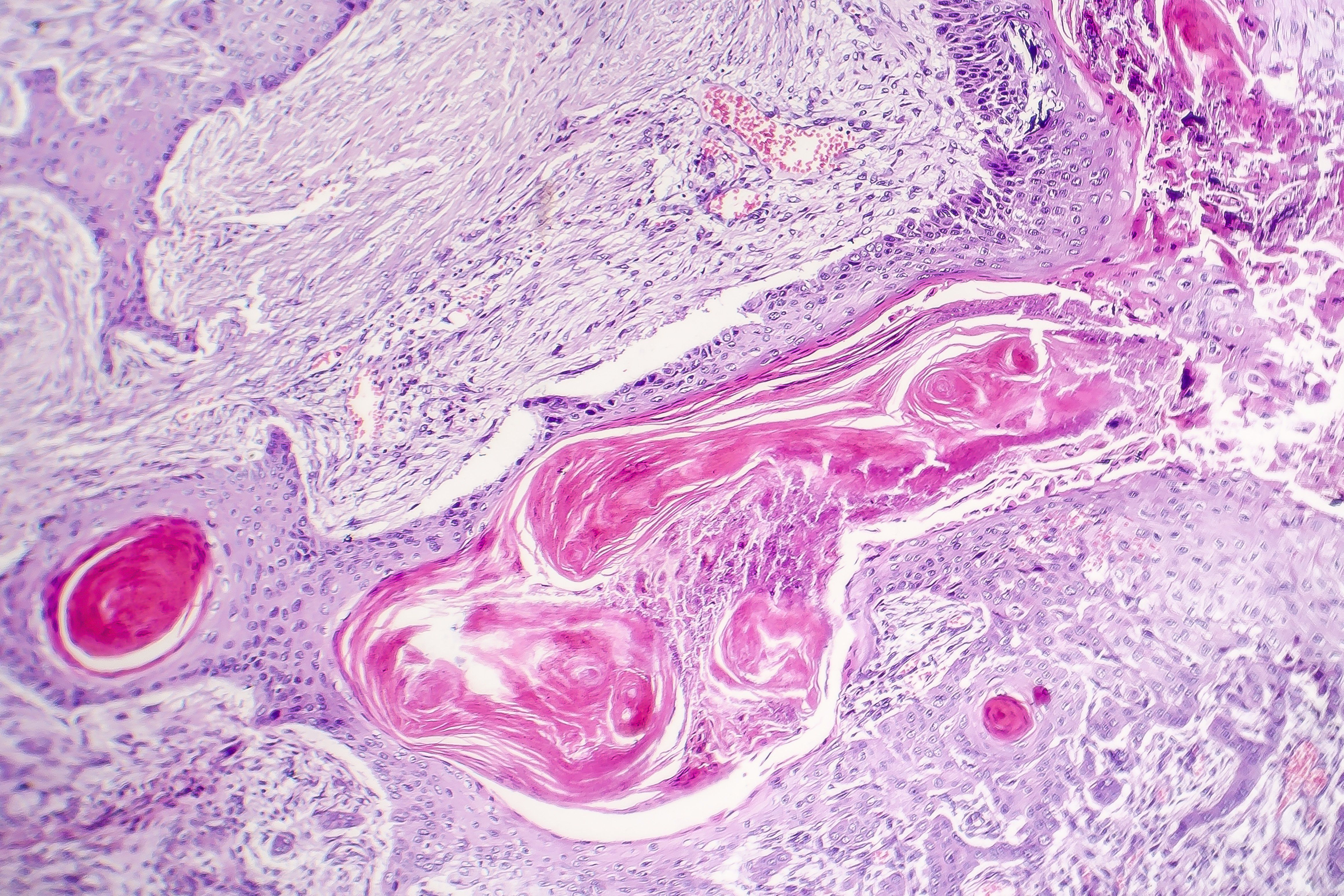
The epidermal growth factor (EGFR) inhibitor cetuximab (Erbitux, Eli Lilly) achieved good outcomes in patients with advanced SCC, according to results of a recent study.1
RELATED: Immunotherapy for advanced squamous cell carcinoma
Following basal cell carcinoma, cutaneous SCC is the most common skin cancer in western countries and their incidence remains on a steady rise. Some of the risk factors for SCC include chronic sun exposure, skin that is sensitive to ultraviolet radiation, and immunosuppression, which, in part, can explain why the geriatric population is more susceptible to developing this type of nonmelanoma skin cancer.
The recent multicenter study headed by Henri Montaudie, M.D., from the department of dermatology, University Hospital of Nice, Nice, France, found the monoclonal antibody cetuximab to be safe and effective for the treatment of advanced cutaneous SCC. In the retrospective study that included 58 patients (median age: 83.2 years) with advanced SCC, researchers evaluated the clinical outcomes of cetuximab administered as a monotherapy. The majority of patients (over 90%) were chemotherapy naïve and the median follow-up was 11.7 months. The primary endpoint was the disease control rate (DCR) at six weeks according to the RECIST (response evaluation criteria in solid tumors) criteria. Secondary endpoints included DCR at 12 weeks, objective response rate (ORR) at six and 12 weeks, progression-free survival (PFS), overall survival (OS), and safety profile.
Study outcomes showed that the DCR was 87% and 70% at six and 12 weeks, respectively, while the ORR was 53% and 42% at six and 12 weeks, respectively. The median PFS and OS were 9.7 months and 17.5 months, respectively. Data showed that 51 patients (88%) experienced toxicity, and 67 adverse events related to cetuximab occurred, with the large majority of them (84%) having a severity of grade 1 or 2.
“Cetuximab proves to be safe and efficient for the treatment of patients, even elderly ones, with advanced cutaneous SCC. These results indicate that cetuximab is a promising agent to test in new combinations, especially with immune checkpoint inhibitors such as anti-PD-1 agents,” wrote Henri Montaudie, M.D., and colleagues, in the study recently published in Oncotarget.
The vast majority of SCC lesions are treated with surgical resection and have a cure rateexceeding 90 percent in early-stage disease. In stark contrast however, the 5-year overall survival rate is below 50% for patients with local lymph node metastases and less than 10 percent for those with distant metastases. In more advanced cases, chemotherapy and radiotherapy are usually employed, albeit in a palliative systemic therapy setting, underscoring the urgency to find more effective therapies for locally advanced or metastatic SCC.
RELATED: A paradigm shift in squamous cell carcinoma treatment
Recent trials2,3 with the PD-1 inhibitor cemiplimab for the treatment of inoperable cutaneous SCC showed response rates around 50%, with durable responses.
“It is very likely that this immunotherapy will quickly become the first-line treatment for advanced SCC, and it will be certainly interesting to combine it with cetuximab,” the study authors wrote.
Although the commonly used cisplatin-based combination chemotherapies may achieve an overall response rate of up to 80%, the efficacy is usually not durable. Moreover, the use of chemotherapy is limited due to the many adverse events, especially in elderly patients, who are the largest population of concern for cutaneous SCC.
According to the researchers, the study not only confirms the efficacy of cetuximab used as a first-line agent in advanced SCC cases, but also demonstrates an acceptable tolerance among these patients.
“Cetuximab may be considered as a therapeutic option in this setting, particularly for elderly patients in whom chemotherapy is not appropriate. Several clinical trials have shown that anti-PD-1 agents are active in cutaneous SCC. Further clinical evaluations are needed to determine the role of cetuximab and immune checkpoint inhibitors in combination,” the study authors wrote.
Disclosures:
Dr. Montaudie is a principal investigator in studies conducted by Bristol-Myers Squibb, MSD Pharma (Merck & Co., d.b.a Merck Sharp & Dohme). Research grant to institution received Bristol-Myers Squibb, LeoPharma.
References:
1. Montaudie H, et al. Cetuximab is efficient and safe in patients with advanced cutaneous squamous cell carcinoma: a retrospective, multicentre study. Oncotarget. 2020 Jan 28;11(4):378-385. doi: 10.18632/oncotarget.27434. eCollection 2020 Jan 28.
2. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018; 379:341351. 10.1056/NEJMoa1805131.
3. Sidaway P. Cemiplimab effective in cutaneous SCC. Nat Rev Clin Oncol. 2018; 15:472. 10.1038/s41571-018-0056-5





