- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
80% of Patients With AD Demonstrated Sustained Skin Clearance With Lebrikizumab in 2-Year Data
Author(s):
Skin clearance, itch relief, and reduced severity of atopic dermatitis were sustained with monthly maintenance dosing.
Rochu_2008/Adobe Stock
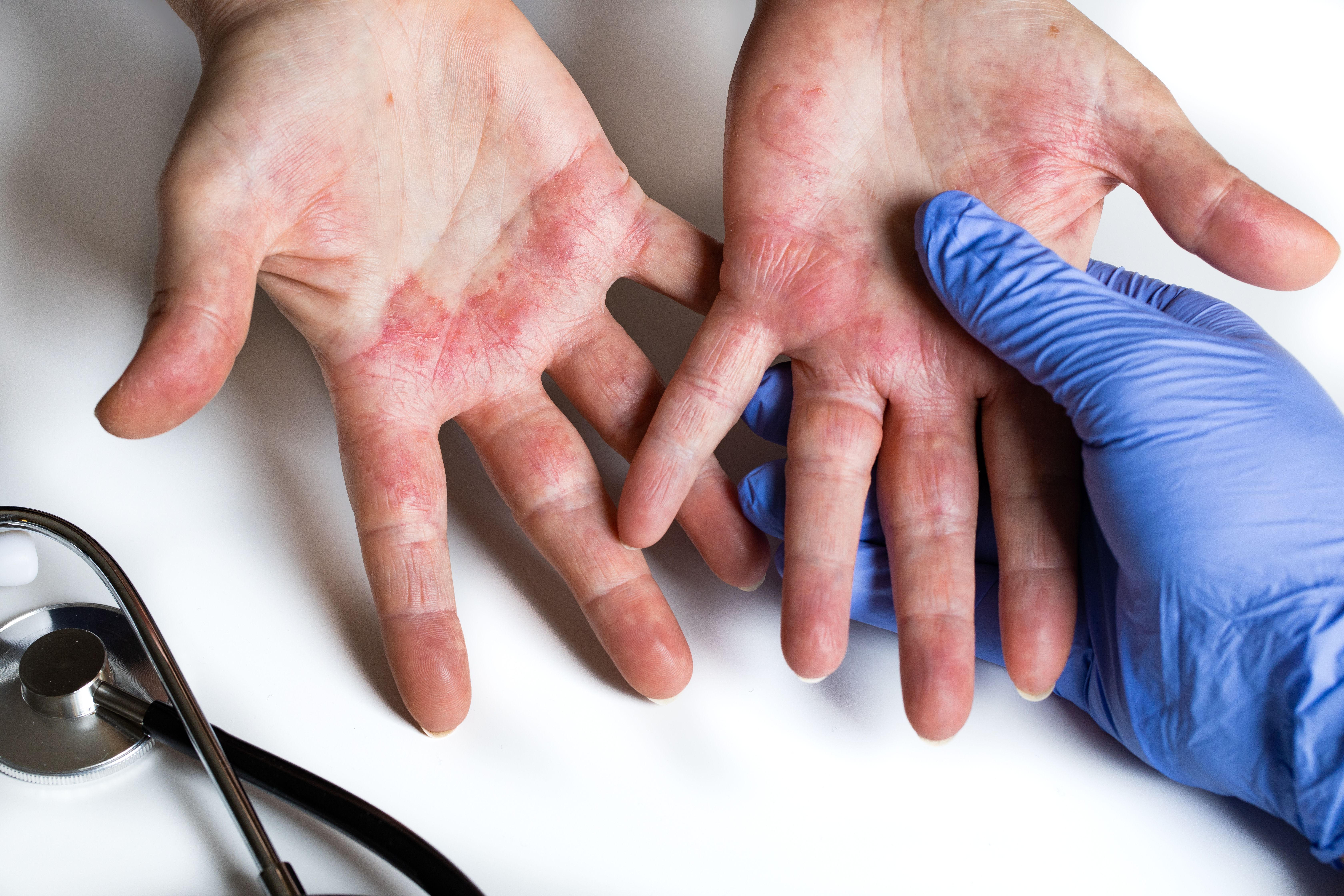
Nearly 80% of patients with moderate to severe atopic dermatitis (AD) who were treated with maintenance dosing of lebrikizumab (Eli Lilly) demonstrated sustained skin clearance, itch release, and reduced severity of disease after 2 years, according to newly-released data.1
Data comes from the ADjoin study, a long-term extension of the ADvocate 1 and ADvocate 2, which evaluated the efficacy of lebrikizumab as a monotherapy, and ADhere, which examined lebrikizumab's efficacy in combination with topical corticosteroids.
The ADjoin study further explored the long-term efficacy of lebrikizumab among patients from the ADvocate and ADhere trials who had achieved an Investigator's Global Assessment (IGA) score of 0 or 1 or an Eczema Area and Severity Index 75% improvement (EASI-75) by week 16 of treatment with lebrikizumab.
In the long-term extension study, patients received 250 mg of lebrikizumab either biweekly or on a monthly basis.
Read Dermatology Times' previous coverage of lebrikizumab here.
Patients who had previously participated in the ADvocate trials (n=181) and patients who participated in the ADhere trial (n=86) demonstrated sustained clearance as measured by IGA, EASI, and pruritus numerical rating scale (NRS) score.
Among patients receiving monthly maintenance doses of lebrikizumab, 76% from the ADvocate trials and 79% from ADhere exhibited IGA scores of 0 or 1, indicative of complete or nearly complete skin clearance. In patients treated with lebrikizumab on a biweekly basis, 86% and 84% in the ADvocate and ADhere trials achieved IGA 0 or 1, respectively.
The majority of patients receiving either monthly or biweekly maintenance doses achieved EASI-75 or EASI-90. Furthermore, 90% of patients among cohorts receiving lebrikizumab on a monthly basis achieved a minimum 4-point improvement in pruritus NRS score. 100% of prior participants from the ADvocate trials, while 82% of prior participants from the ADhere trials achieved the same level of itch reduction.
"Lebrikizumab, administered with a once-monthly dose following an induction phase, demonstrated efficacy for patients with moderate-to-severe atopic dermatitis, offering sustained relief from some of the most distressing signs and symptoms of the disease," said Emma Guttman-Yassky, MD, PhD.1 Guttman-Yassky is the Waldman Professor and Health System Chair at the Department of Dermatology, Director of the Center of Excellence in Eczema and the Laboratory for Inflammatory Skin Diseases at the Icahn School of Medicine at Mount Sinai in New York, and the senior author and investigator of the ADjoin long-term analysis.
"Long-term data is critical for healthcare providers making treatment decisions with patients. These impressive two-year data underscore the lasting impact this potential first-line biologic treatment option may provide for people living with this disruptive disease," she said.
Lebrikizumab's safety profile was consistent with consistent studies of the drug in this patient population and indication. Approximately 62% of patients reported mostly mild or moderate adverse events, including conjunctivitis, shingles, and injection site reactions.
"Results from ADjoin reinforce the strong efficacy and safety profile of lebrikizumab seen in the other Phase 3 atopic dermatitis trials. These data also further our understanding of the long-lasting benefits of lebrikizumab as a potential first-line biologic treatment for patients," said Lotus Mallbris, MD, PhD, senior vice president of global immunology development and medical affairs at Lilly.1 "We look forward to working with global regulatory authorities to bring this important medicine to patients."
Reference
- Nearly 80% of patients with moderate-to-severe atopic dermatitis maintained clear or almost clear skin with Lilly’s lebrikizumab monthly maintenance dosing at two years. Eli Lilly and Company. October 20, 2023. Accessed October 20, 2023. https://investor.lilly.com/news-releases/news-release-details/nearly-80-patients-moderate-severe-atopic-dermatitis-maintained#:~:text=INDIANAPOLIS%20%2C%20Oct.%2020%2C%202023,demonstrated%20in%20the%20ADjoin%20long%2D
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









