- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
A Highly Targeted Approach to Treating Atopic Dermatitis
Created and funded by LEO Pharma Inc.

Andrew F. Alexis, M.D., MPH
Professor of Clinical Dermatology
Weill Cornell Medicine
Dr. Alexis is an ECZTRA 3 clinical trial investigator and paid consultant of LEO Pharma Inc.
Atopic dermatitis (AD) arises from a complex pathophysiology, including environmental factors, genetic influences, acute and chronic inflammation, dysbiosis, and a perpetual itch-scratch cycle.1-3 Several Th2 cytokines, including interleukin IL-13 are drivers of inflammation in AD.4,5,6 IL-13 in particular, can offer a vital point of intervention for management of this chronic disease.5
IL-13: a driver of inflammation in atopic dermatitis.
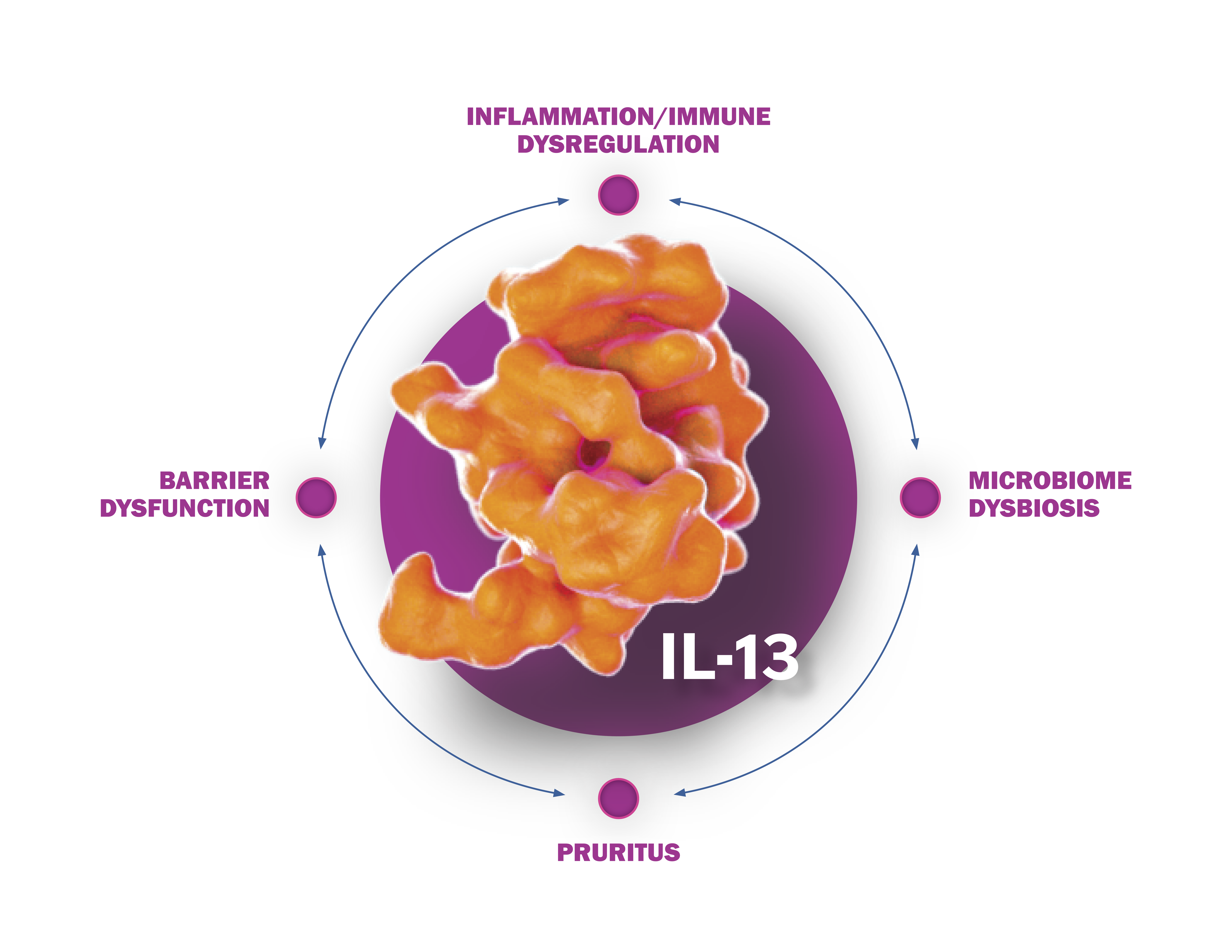
“As an integral part of the Th2 pathway that is central to the pathogenesis of AD, targeting IL-13 has been shown to help reduce clinical and molecular hallmarks of AD,” said Andrew F. Alexis, MD, MPH, Professor of Clinical Dermatology, Weill Cornell Medicine. “In my experience as a healthcare professional, I have seen patients who suffer from inadequate disease control experience meaningful improvements in their AD with the use of targeted treatments.”
Unmet Need in AD
More than half of AD patients worry over an impending flare, yet non-adherence to treatment plans is common, suggesting there is a need for improvement. There have been developments in the understanding of factors playing a role in the immune and inflammatory processes underlying AD signs and symptoms that will help improve the treatment landscape.7-10
“AD is a persistent, unpredictable disease,” said Dr. Alexis. “Many adults with AD have difficulty maintaining adequate disease control, struggling with skin lesions and itching. There is a need for additional treatments when topical Rx therapies only go so far.”
Given the promise of IL-13-specific inhibition, a biologic treatment that specifically targets this cytokine helps adult patients with AD and their clinicians see lasting disease control.
“We are fortunate to have proven targeted treatment options for patients with AD that provide long-term control and disease management, allowing clinicians to address challenges patients may be having with their AD,” said Dr. Alexis.
A Treatment That Exclusively Targets IL-13
Adbry™ (tralokinumab-ldrm), which received U.S. Food and Drug Administration (FDA) approval in December 2021, is the first and only biologic developed to specifically target and neutralize IL-13.5,11 Adbry is a fully human, high-affinity monoclonal antibody that selectively inhibits IL-13 interaction with the type II receptor complex, preventing IL-13-induced inflammatory responses in the skin.12 It is indicated for the treatment of adults with moderate-to-severe AD whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Adbry, which is not a steroid or an immunosuppressant, can be used with or without topical corticosteroids (TCS) and has no boxed warning in the Prescribing Information.11
Adbry selectively inhibits IL-13 interaction with the type II receptor complex (IL-13Rα1/IL-4Rα), preventing IL-13–induced inflammatory responses in the skin.
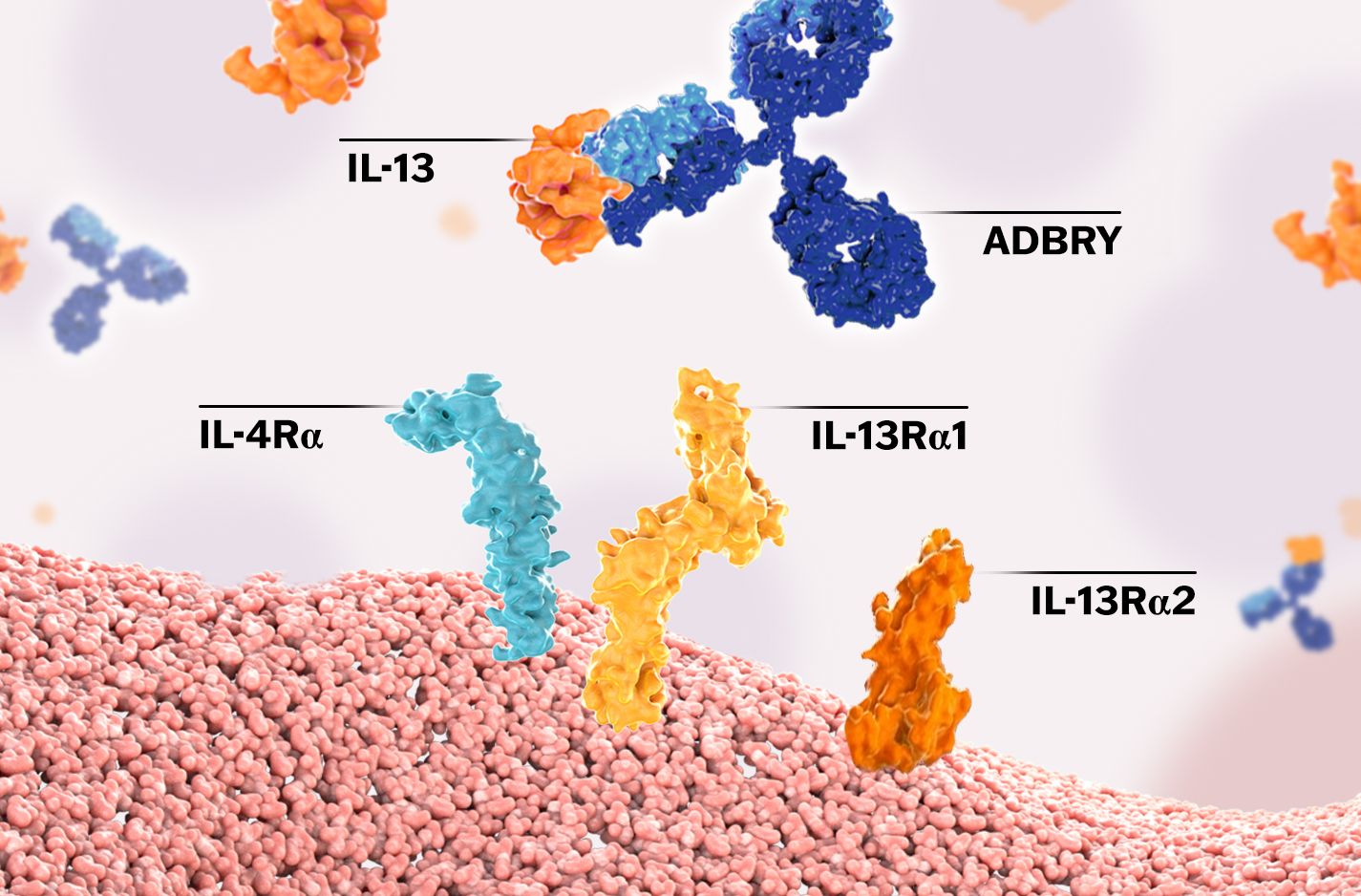
With FDA approval of Adbry, clinicians and adult patients have a targeted biologic treatment option for the management of this chronic disease. It has demonstrated visible improvements in skin lesions and itch relief among patients in clinical trials at Week 16.5,11
Adbry was evaluated in three robust pivotal trials in nearly 2,000 adult patients with moderate-to-severe AD. Its approval was based on safety and efficacy results from the pivotal Phase 3 ECZTRA 1 and 2 monotherapy trials and ECZTRA 3 combination therapy with concomitant TCS as needed trial. In all three pivotal trials, Adbry 300 mg Q2W alone or with TCS as needed met the primary endpoints at Week 16, as measured by an Investigator Global Assessment score of clear or almost clear skin (IGA 0/1) or at least a 75% improvement in the Eczema Area and Severity Index score (EASI-75). Improvements in the secondary endpoint of itch reduction (≥4-point reduction in Worst Daily Pruritus NRS weekly average) also were observed at Week 16:11
Example improvement in EASI from baseline to Week 16 in patient receiving Adbry in ECZTRA 2. Individual results may vary.
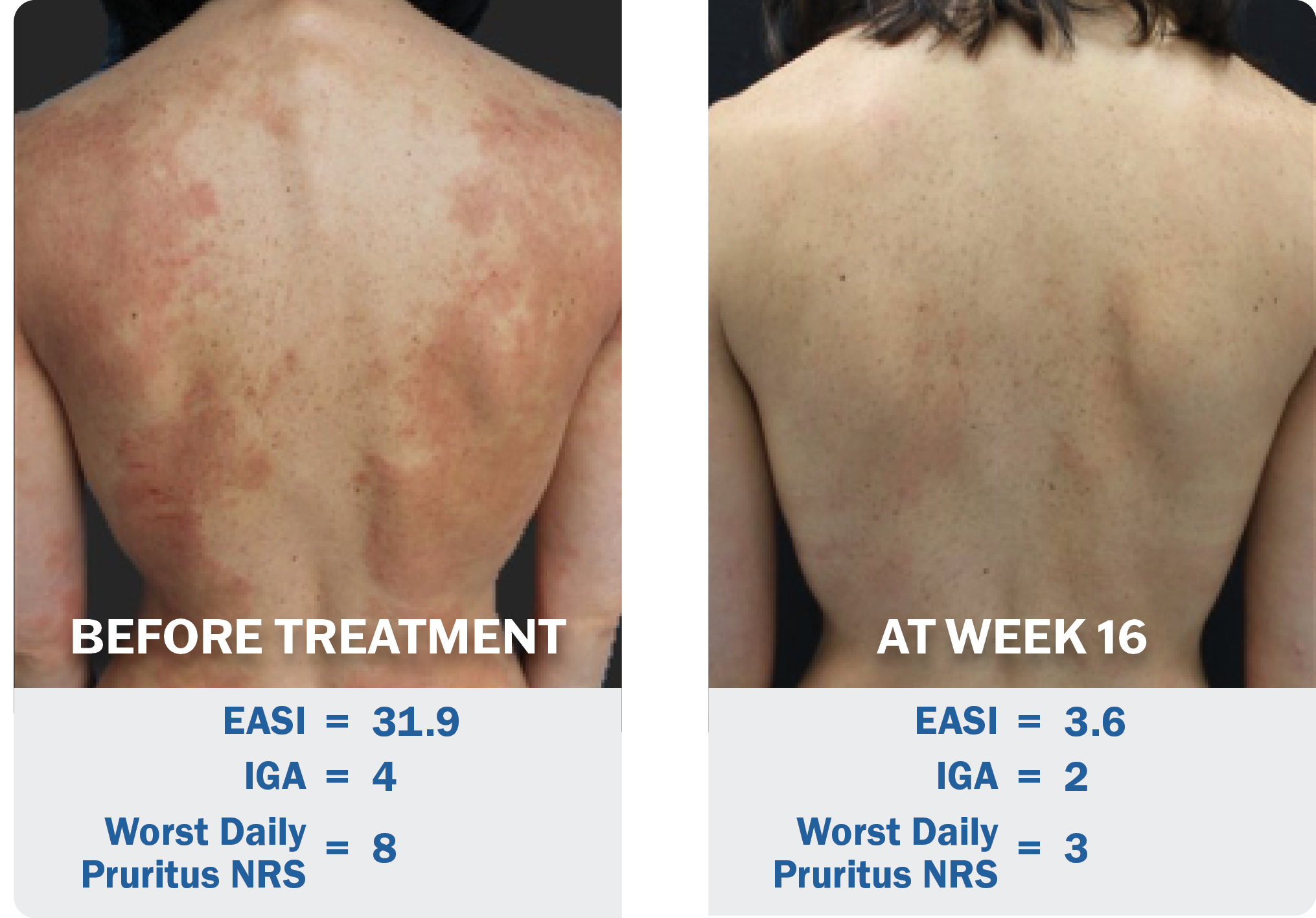
- IGA 0/1: In ECZTRA 1 and 2, respectively, 16% and 21% of patients treated with Adbry 300 mg Q2W achieved IGA 0/1 at Week 16 vs 7% and 9% with placebo (P=0.002 and P<0.001, respectively). In ECZTRA 3, 38% of patients treated with Adbry 300 mg Q2W plus TCS achieved IGA 0/1 at Week 16 vs 27% with placebo plus TCS (P=0.033).13,14*
- EASI-75: In ECZTRA 1 and 2 respectively, 25% and 33% of patients treated with Adbry 300 mg Q2W achieved EASI-75 at Week 16 compared with 13% and 10% (P<0.001 for both trials). In ECZTRA 3, 56% of patients treated with Adbry 300 mg Q2W plus TCS achieved EASI-75 at Week 16 vs 37% of patients with placebo plus TCS (P<0.001).13,14*
- Worst Daily Pruritus NRS (weekly average): In ECZTRA 1 and 2, respectively, 20% and 25% of patients treated with Adbry 300 mg Q2W experienced itch reduction at Week 16 vs 10% and 9% with placebo (P=0.002 and P<0.001, respectively). In ECZTRA 3, 46% of patients treated with Adbry 300 mg Q2W plus TCS experienced itch reduction at Week 16 vs 35% with placebo plus TCS (P=0.039).13,14*
Long-Term Efficacy and Safety
Clinical trials have also evaluated long-term efficacy and safety of Adbry to help patients and clinicians achieve lasting disease control. According to clinical trial results:
- Nearly 9 out of 10 responders at Week 16 treated with Adbry Q2W plus TCS as needed experienced lasting disease control at Week 32* as reported in ECZTRA 3.11*§
- More than half of responders at Week 16 treated with Adbry Q2W maintained IGA 0/1 and EASI-75 at Week 52, without any TCS use in a pooled analysis of ECZTRA 1 and 2 trial data.14*^
Adbry demonstrated long-term safety in five extensive clinical trials through 52 weeks. In ECZTRA 1, 2 and ECZTRA 3, the most common adverse events with Adbry (incidence ≥1% and greater than placebo) at Week 16 were upper respiratory tract infections (mainly reported as common cold), conjunctivitis, injection site reactions, and eosinophilia.11 In ECZTRA 1 and ECZTRA 2, the frequency of adverse reactions during maintenance treatment with Adbry 300 mg Q2W and Q4W, respectively, was 44% and 34%; in ECZTRA 3, it was 43% and 26%, respectively, with Adbry 300 mg Q2W and Q4W plus TCS as needed.11 In a pooled analysis of five trials, the incidence of conjunctivitis was 7.5% for Adbry (n=1,582) vs 3.1% for placebo in the initial treatment period up to Week 16.11 Two cases of conjunctivitis led to discontinuation.14
Adbry is the only biologic for AD with a reduced maintenance dosing option, providing the flexibility to treat with Q2W or Q4W dosing after 16 weeks of treatment for patients achieving clear or almost clear skin, and who are less than 100 kg.11 To date, Adbry has been prescribed to more than 6,500 appropriate patients in the U.S.
Discover why Adbry can be your first-choice biologic for moderate-to-severe AD when topical Rx therapies only go so far. For additional information, visit https://www.adbryhcp.com/.
*Subjects who received rescue treatment or with missing data were considered non-responders.
§Responders were defined as subjects with an IGA 0 or 1 (“clear” or “almost clear”) or EASI-75 at Week 16. At Week 16, responders were re-randomized to Adbry 300 mg Q2W plus TCS or Adbry Q4W plus TCS for another 16 weeks.
^Responders were defined as subjects with an IGA 0 or 1 (“clear” or “almost clear”) or EASI-75 at Week 16. At Week 16, responders were re-randomized to Adbry 300 mg Q2W, Adbry Q4W, or placebo every other week for another 36 weeks.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.





