- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Acne, Skin Appearance Improved With Salicylic and Lipohydroxy Acids
Author(s):
Researchers said a facial serum and mask containing both acids resulted in statistically significant improvements to the skin.
A facial serum and mask containing both salicylic and lipohydroxy acids was both safe and effective in producing statistically significant improvements to the skin, including indications such as acne, hydration, and sebum secretion.
Bungon/AdobeStock
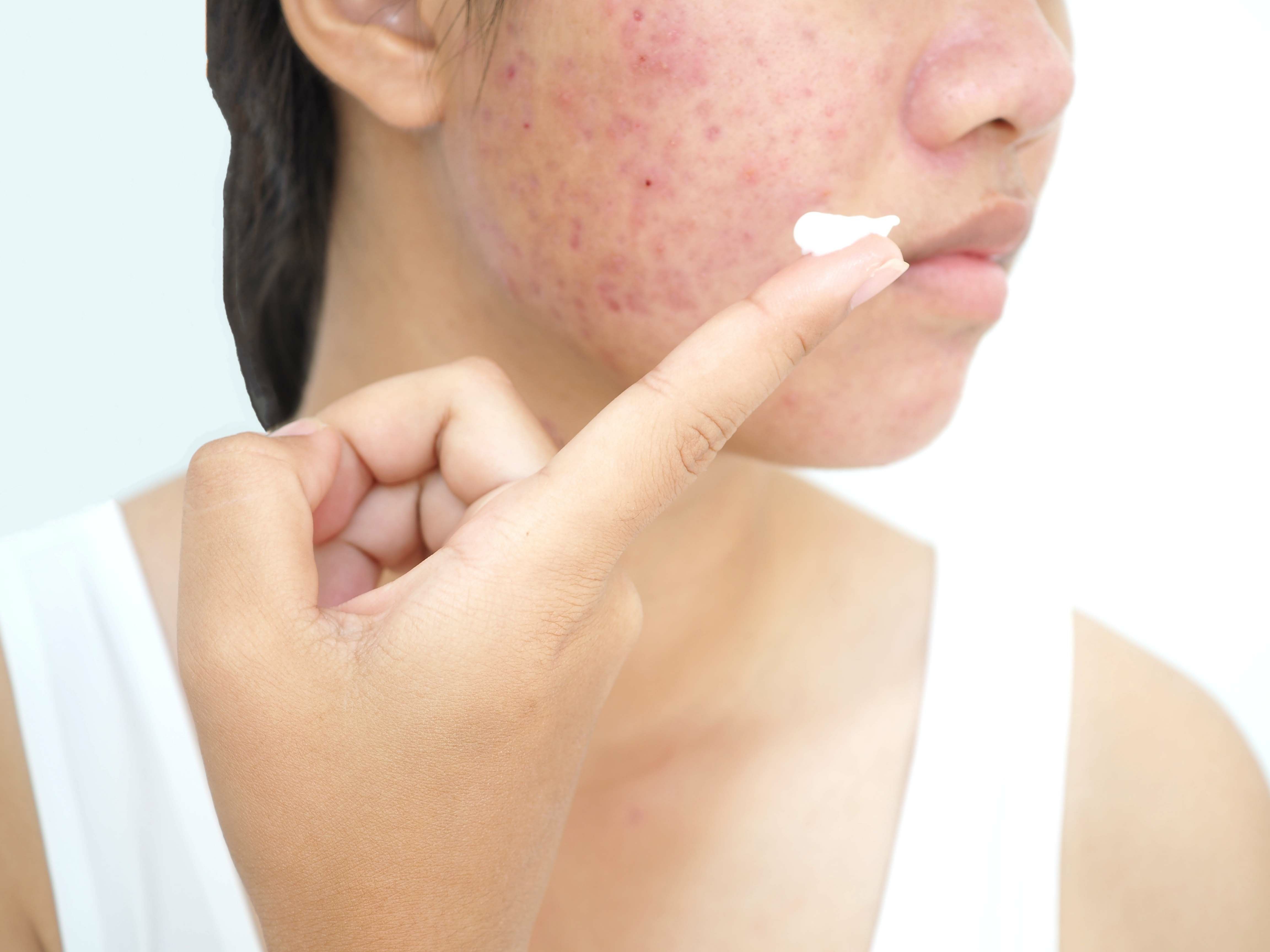
In a recent study,1 researchers sought to evaluate the safety and effectiveness of both a serum and mask containing salicylic and lipohydroxy acids in patients with various skin conditions, including comedones, post-inflammatory erythema (PIE), and/or hyperpigmentation, in addition to sebum dysregulation.
Previous studies, they found, reported that lipohydroxy acid significantly decreased both the size and number of microcomedones in patients with acne and acne-prone skin. Additionally, prior research is indicative of salicylic acid’s effectiveness in acne vulgarius and post-inflammatory hyperpigmentation (PIH). In other trials, combining several acidic ingredients proved to be efficacious in controlling sebum production and improving PIH.
Adults ages 18 to 45 with the following criteria were eligible for participation:
- Grade 3 or greater pore visibility
- Greater than or equal to 8 non-inflammatory facial acnes
- Greater than or equal to 10 blackheads on the nose
- Inflammatory and/or non-inflammatory acne lesions
- Self-assessed sebum dysregulation
- Visible symptoms of PIE and/or PIH
Certain factors such as the use of several topical or oral treatments, other dermatologic conditions affecting the test areas, a history of skin cancer or malignant melanoma, and more, were grounds for exclusion from participation.
A total of 93 trial participants were first randomized and assigned to either the serum treatment group or to the serum and mask treatment group.
Participants in the serum group (n=48) were asked to use a cleanser and moisturizer—with a sunscreen in the morning—in addition to applying the serum on a twice-daily basis.
Participants in the serum and mask group (n=45) used a cleanser, moisturizer, and sunscreen at the same rates as listed above. However, from days 0 to 6, they applied the mask once every 2 days. From days 9 through 54 of the trial, they were asked to apply it once every 3 days. The mask was used before the serum and worn for 10 to 15 minutes at a time.
The dermatologist responsible for blindly assigning participants to each group also completed participant assessments at days 0, 1, 7, 14, 28, and 56. In these assessments, all participants were evaluated using the investigator global assessment (IGA) scale. Using the visual analogue scale (VAS), all participants were assessed for PIE and PIH intensity, as well as overall skin tone evenness.
Additionally, the dermatologist counted the number of visible closed comedones, open comedones, papules, pustules, and PIE/PIH foci.
Several other factors, including skin pore density, forehead sebum secretion, skin hydration, transepidermal water loss, and the percentage of facial area where participants had blackheads on their nose, were also assessed.
On each side of participants’ faces, a trained technician photographed facial areas using cross-polarized, parallel polarized, and ultraviolet light.
At days 14 and 56, all participants completed a self-assessment questionnaire for researchers to additionally analyze product efficacy.
By the conclusion of the study, 41 participants in the serum group and 42 participants in the serum and mask combination group had fully completed the trial and analysis.
At week 8, the combination treatment group experienced a statistically significant improvement in IGA score from baseline, whereas the serum-only group did not.
Participants in both groups experienced statistically significant improvements in skin pore density, and skin tone evenness. At weeks 2 and 4, both groups had significant improvements to the number of closed facial comedones and open nasal comedones. Additionally at week 2, both groups experienced improvements to PIE and PIH intensity from baseline. The number of PIE foci on the nose and PIH foci on the face were also reduced at week 8.
From baseline to weeks 2 and 4, both groups experienced improvements to sebum secretion and skin hydration. Transepidermal water loss improved significantly by week 8 in the group assigned to the serum.
“A difference in efficacy was noted at some points between the two groups. Particularly, at T56d, acne severity in the Serum + Mask group was significantly improved compared with baseline, and the improvement was significantly more pronounced than that of the Serum group," study authors wrote. “In addition, the number of closed comedones on the face was improved in the Serum + Mask group compared with the Serum group at this time point. The improvement of skin tone evenness from baseline was faster in the Serum + Mask group than in the Serum group, starting at 1 and 8 weeks post-treatment, respectively. At T56d, the improvement in PIH intensity was significantly more pronounced in the Serum + Mask group than in the Serum group."
In both groups, participants did not report adverse effects or skin discomfort at any point during the study.
Study limitations included the lack of use of the Global Evaluation Acne scale and the fact that the study was not designed to be split-face controlled.
“In conclusion, using the study serum for 8 weeks improved the skin condition by regulating skin barrier function and achieving a balance of skin hydration and sebum secretion, removing acne and closed comedones, and reducing PIE and PIH intensities,” study authors wrote. “Addition of the mask further improved closed comedones, skin tone evenness and acne severity, and accelerated the therapeutic effects without compromising safety.”
Reference
- Li S, He X, Zhang Z, et al. Efficacy and safety of a facial serum and a mask containing salicylic acid and lipohydroxy acid in acne management: A randomized controlled trial. Journ of Cosmetic Dermatol. 2023. doi:10.1111/jocd.15746
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











