- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Afamelanotide for vitiligo to be tested in international clinical trial
The addition of afamelanotide, known by the trademark Scenesse (Clinuvel), may represent a major advance in the treatment of vitiligo, a condition for which there is no therapy in the United States that has been approved by the Food and Drug Administration.

Key Points

"There is an enormous unmet global need in treating patients with vitiligo," says Pearl Grimes, M.D., an investigator at one of three sites in the United States that is participating in an international study examining the impact of afamelanotide treatment on patients with vitiligo.
"It's a chronic disease. It's not life-threatening, but it sure is life-altering," she says. "This study is going to address an unmet need for a psychologically and emotionally devastating disease that affects self-esteem and causes depression."
The International Scenesse Pilot Repigmentation Evaluation (INSPIRE) program, a pilot phase 2 clinical trial, is examining the impact of afamelanotide in patients diagnosed with vitiligo.
The open-label trial is designed so all patients at six sites - with international sites in France, Italy and Switzerland - will be treated with narrowband ultraviolet B (NB-UVB) radiation three times weekly for six months, totaling 72 treatments altogether.
A total of 120 patients are scheduled to be enrolled in the trial, with half of the patients receiving Scenesse in addition to NB-UVB every 28 days over six months. Investigators will compare the impact of the two treatment regimens at the end of the treatment period and continue to follow patients for another six months, with three clinical visits scheduled, to assess repigmentation effects of the two regimens.
"If we see that the combination of narrowband UVB and afamelanotide is superior to narrowband UVB as a monotherapy, then it gives us pilot primary data to move forward in larger studies," Dr. Grimes says.
If the combination arm proves superior to NB-UVB monotherapy, she says, afamelanotide may be compared as a monotherapy against NB-UVB monotherapy.
"If the efficacy of the drug is limited as a monotherapy, but we find in combination studies that we can shorten the treatment times with NB-UVB, that would be a substantial advance," Dr. Grimes says. Future studies of the treatment would include larger numbers of patients.
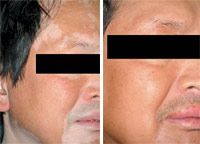
Available therapies
Therapies that are administered in the United States to treat vitiligo are administered in an off-label fashion.
The use of NB-UVB radiation has become a common therapy for this condition and it has demonstrated efficacy, but it may not address vitiligo that is very extensive, according to Dr. Grimes, who is director of the Vitiligo and Pigmentation Institute of Southern California, and clinical professor of dermatology, University of California at Los Angeles. In addition, she says, some patients with the condition are unresponsive to NB-UVB therapy.
"It's appropriate (NB-UVB) if there is more than 10 to 15 percent body surface area affected," Dr. Grimes says. "If patients have extensive areas of the body surface affected by vitiligo, then those patients may need an additional stimulus for repigmentation."
The side effect profile of afamelanotide is favorable, with patients reporting side effects such as nausea, headaches and dizziness, but no side effects that would lead to treatment withdrawal, according to Dr. Grimes.
"I think the most common thing that patients indicate is the immediate pigment darkening in their normal skin," Dr. Grimes says. "Patients have said it happens in less than 48 hours."
Dr. Grimes says afamelanotide has been explored to treat other conditions, such as erythropoietic protoporphyria, which causes patients to experience profound sensitivity.





