- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Allergan Announces Positive Topline Results From Studies of TrenibotulinumtoxinE for Glabellar Lines
Author(s):
BoNT/E represents a novel approach in the world of neurotoxins, characterized by its rapid onset of action.
Denis/Adobe Stock
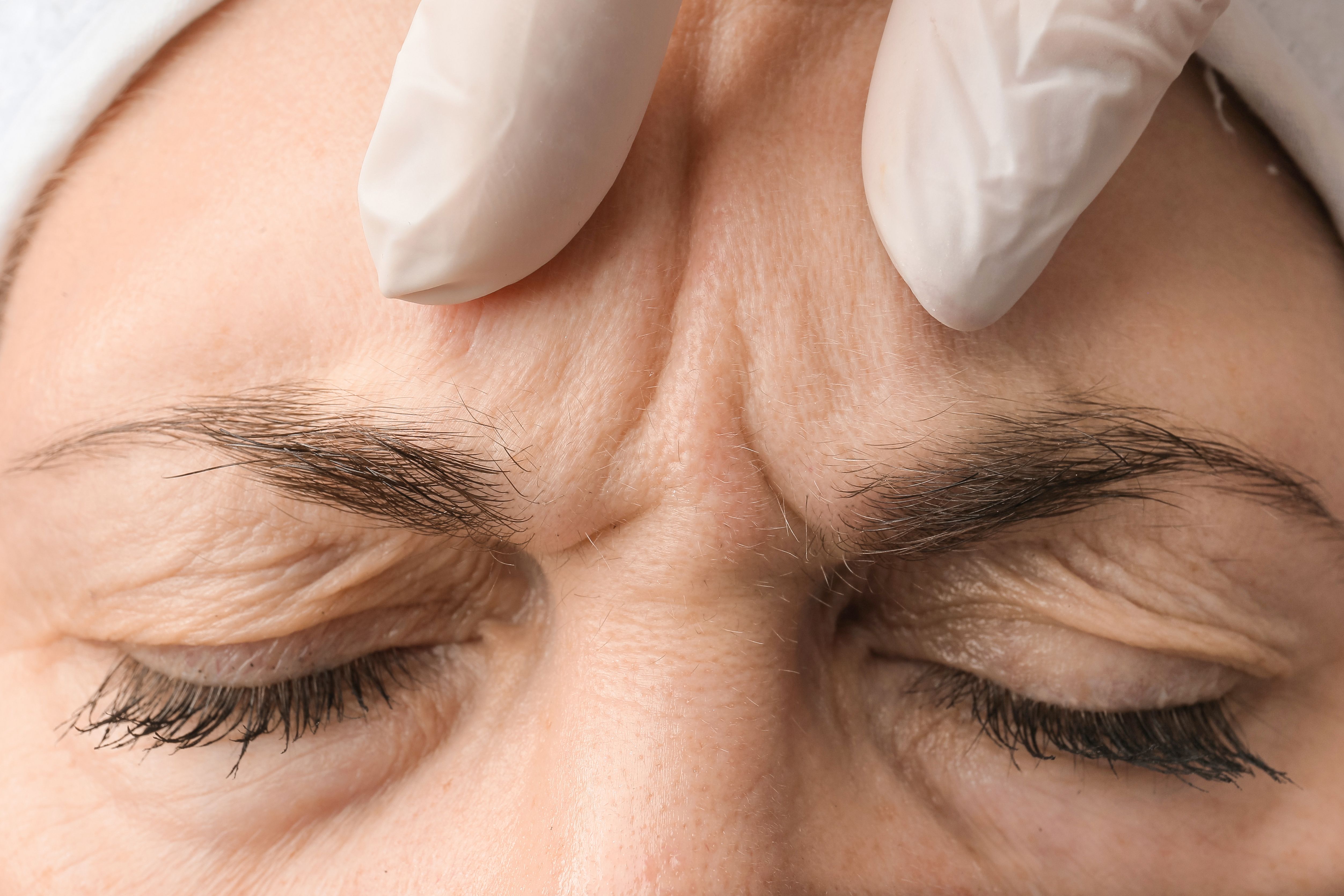
Allergan Aesthetics, an AbbVie company, has announced positive topline results1 of two pivotal phase 3 clinical trials for trenibotulinumtoxinE (BoNT/E). The trials, named M21-500 and M21-508, focused on the treatment of moderate to severe glabellar lines and have shown remarkable promise.
The phase 3 trials, carried out across multiple centers, encompassed 947 adult patients from the United States, Canada, and Europe, all with moderate to severe glabellar lines between the eyebrows. Participants included those who were toxin-naïve and those who had prior experience with neurotoxin treatments. The evaluation period spanned 12 weeks, with each subject receiving up to two treatments of BoNT/E.
BoNT/E represents a novel approach in the world of neurotoxins, characterized by its rapid onset of action, which was observed as early as 8 hours after administration, and a short duration of effect, spanning 2-3 weeks. The results of both phase 3 studies demonstrated significant improvements in glabellar line severity, with statistical significance in favor of BoNT/E compared to a placebo. These results extended to secondary endpoints, including patient-reported outcomes on overall treatment satisfaction.
Darin Messina, PhD, senior vice president of aesthetics R&D at AbbVie, said,1 "BoNT/E is a first-in-class, short-acting neurotoxin in development, and these results demonstrate its potential to bring true innovation to the aesthetics industry."
"Treatment with a product differentiated by a rapid onset of effect and short duration of action could offer these patients a different option compared to currently available neurotoxins," said Cheryl Burgess, MD, FAAD, lead clinical investigator for one of the phase 3 studies.1
The safety profile of BoNT/E is also promising, with treatment-emergent adverse events comparable to placebo, whether administered as a single treatment or consecutively.
Further study results will be presented at upcoming medical meetings, and a phase 3 open-label safety study is underway, with results expected later this year. If approved, BoNT/E would offer a shorter-acting neurotoxin option for patients.
Reference
- AbbVie. Allergan aesthetics announces positive topline results from two pivotal phase 3 studies of Trenibotulinumtoxine (bont/E) for the treatment of glabellar lines. PR Newswire: press release distribution, targeting, monitoring and marketing. October 24, 2023. Accessed October 24, 2023. https://www.prnewswire.com/news-releases/allergan-aesthetics-announces-positive-topline-results-from-two-pivotal-phase-3-studies-of-trenibotulinumtoxine-bonte-for-the-treatment-of-glabellar-lines-301965107.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














