- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Almirall to Assess Patient Wellbeing in New Dermatology Study
Author(s):
This will be the first clinical study in dermatology to assess patients’ wellbeing as a primary endpoint.
fusssergi/AdobeStock
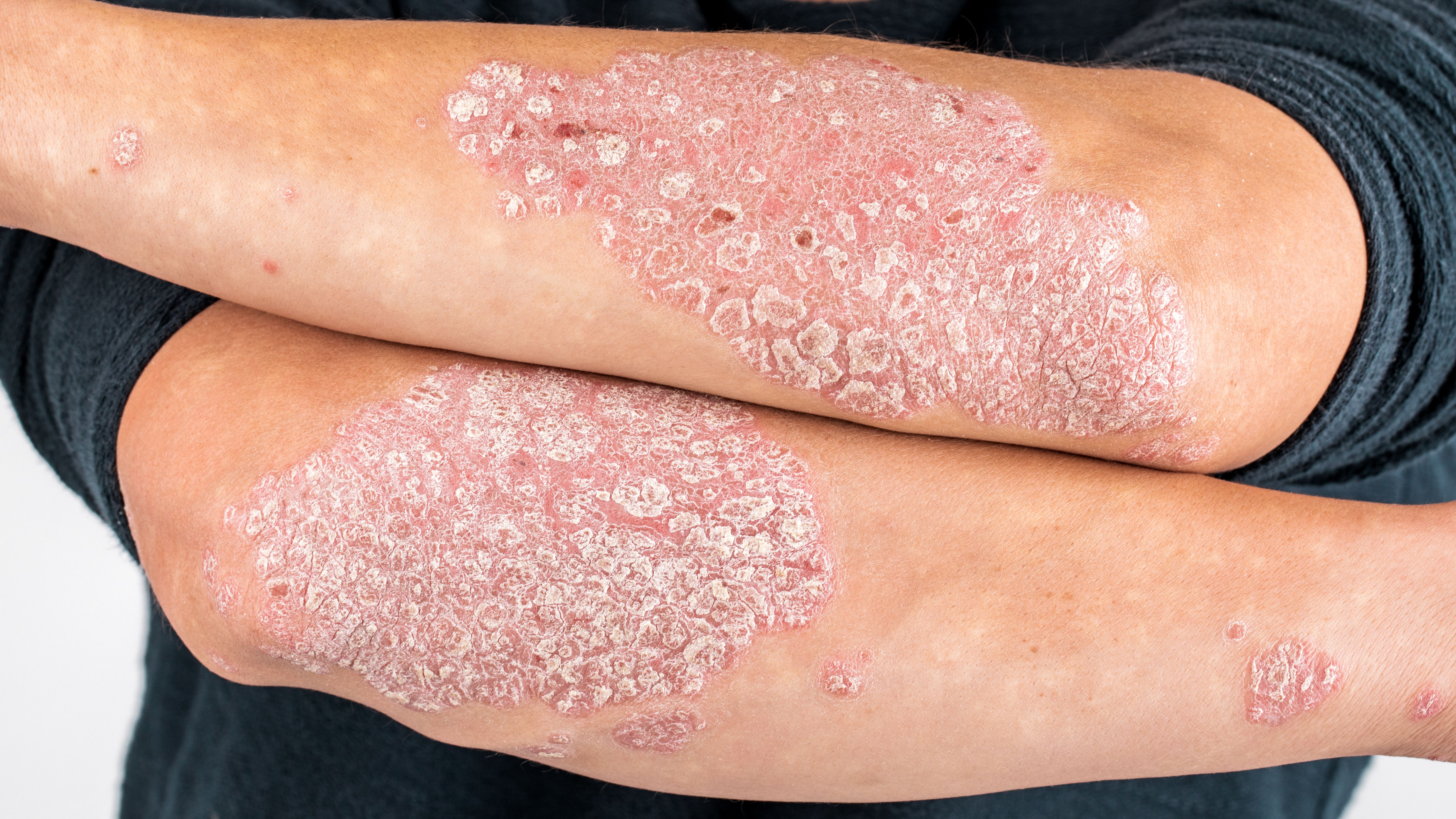
Global biopharmaceutical company, Almirall, S.A., has announced a new study, aimed at capturing patients’ overall wellbeing in a real-world setting, while being treated with tildrakizumab.1
The protocol of the POSITIVE study (published in the British Medical Journal) is using the 5-item World Health Organization Wellbeing Index (WHO-5), a widely used questionnaire that assesses health-related subjective psychological wellbeing in a variety of chronic diseases, but that had never been used in dermatology. Following the holistic approach, The POSITIVE study will also use innovative secondary endpoints, such as evaluating the impact on the family environment, with the FamilyPso questionnaire and on Physician wellbeing, using the Physician’s Satisfaction Score.
This ongoing non-interventional, prospective, observational, real-world evidence study has enrolled approximately 780 adults with moderate-to-severe psoriasis at multiple sites in Europe, including Austria, Belgium, France, Germany, Italy, Spain, Switzerland, The Netherlands, and the United Kingdom. The study will follow these patients for 24 months in their treatment with tildrakizumab. The first 28-week data pull will be presented in scientific meetings at the end of 2023.
Tildrakizumab is a humanized monoclonal antibody that targets the p19 subunit of interleukin-23 (IL-23) and inhibits the release of proinflammatory cytokines and chemokines with limited impact on the rest of the immune system. It is indicated for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy. Researchers in California, Michigan, and New Jersey recently reported that tildrakizumab treatment significantly improved work productivity in psoriasis patients.
Almost 77% of patients believe that psoriasis negatively affects their normal daily activities (personal, social, and work life) and wellbeing and up to 25% of patients have reported being depressed. However, a robust prospective study had never measured the overall wellbeing of these patients.
Matthias Augustin, MD, director of the Institute of Health Care Research in Dermatology and Nursing University of Hamburg and principal investigator of the POSTIVE study, said “We need to go beyond the clinical endpoints and the current use of the DLQI (Dermatology Life Quality Index) questionnaire, extending our understanding of how the patient is really feeling. We need to switch from just looking at the disease burden and setting up positive treatment goals that promote good health and wellbeing. Therefore, our true goal as dermatologists is to reach the maximum wellbeing of our patients. We are really pioneers opening a large terrain of new options in research with the POSITIVE study.”
He added that the results of this study could potentially be added to the available psoriasis evaluation methods and provide dermatologists with new tools to improve their own and their patients’ wellbeing, while enhancing patient-clinician relationships.
Reference
1. Almirall, the first to assess wellbeing in a dermatology clinical study. Almirall. April 2023. https://www.almirall.com/newsroom/news/almirall-the-first-to-assess-wellbeing-in-a-dermatology-clinical-study. Published April 12, 2023. Accessed April 13, 2023.











