- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Alphyn Biologics Shares Positive Interim Results of Phase 2a Trial for AD With Secondary Bacterial Infection
Author(s):
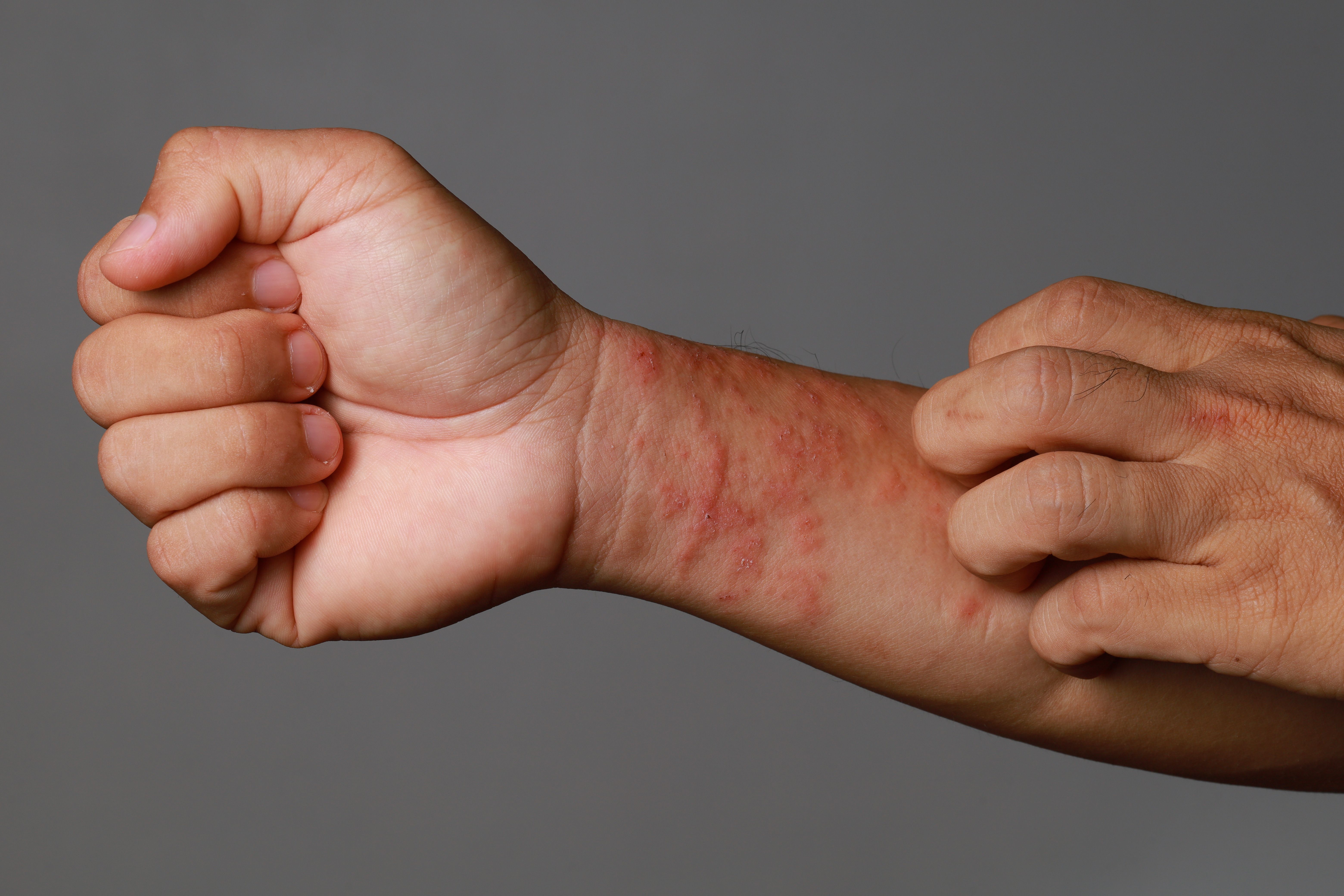
AB-101a is a new topical treatment being investigated for mild, moderate, and severe atopic dermatitis and its immune system component.
Today, Alphyn Biologics announced interim results from the second cohort of its phase 2a clinical trial program of AB-101a, a hydrogel, novel topical candidate for atopic dermatitis (AD). The interim results demonstrated AB-101a is meeting all endpoints for safety and efficacy related to the immune and bacterial components of AD.1
AB-101a clears all AD infections and shows control of the bacterial microbiome on the AD skin to manage flares driven by bacteria in mild, moderate, and severe levels of the disease. For AD's immune system component, AB-101a reduces itch and shows improvement in the Investigator Global Assessment (IGA) and Eczema Area and Severity Index (EASI) AD scores. Results were presented in a poster session at the Society for Pediatric Dermatology annual meeting in Asheville, North Carolina.
The second cohort of the phase 2a trial program is multisite and has completed enrollment. It is expected to conclude with 19 patients. Results from between 7 and 10 patients are included in the interim evaluation.
Interim results include:
- 80% of patients achieved itch score improvement of 4 or greater
- 90% of patients had an EASI score improvement of at least 50%; 44% of patients had an EASI score improvement of 75%
- 40% of patients experienced a reduction of disease severity, as indicated by 2 or greater improvement in the IGA score, with 64% achieving clear or almost clear
- 100% of patients had their infections cleared, and 82% achieved a SIRS (Skin Infection Rating Scale) score improvement of 6 or more points, equal to an improvement of 55 to 75%
- All patients showed a steady decrease in SIRS score, demonstrating control of the AD skin bacterial microbiome and control of the AD flares caused by bacteria
- Effectiveness against bacteria was equivalent to treatment with the gold-standard Vancomycin; one incidence eliminated bacteria resistant to Vancomycin
- Only one Treatment Emergent Adverse Event (TEAE) was reported, showing a strong safety and side effect profile
Earlier this week, Alphyn Biologics also announced the positive results from the phase 2a trial demonstrating significant improvement in skin clearance in AD. AB-101a achieved statistical significance for improvement in IGA scores, with 41 patients in the first cohort reaching clear or almost clear in 4 weeks. The 81% of patients with mild AD population also achieved clear or almost clear skin in 4 weeks, in which improvement is difficult to demonstrate.
Based on the positive trial results, Alphyn Biologics intends to initiate a multinational phase 2b trial with sites in the United States, Europe, Canada, and Australia.
References
- Alphyn Biologics reports encouraging interim results of phase 2a trial for atopic dermatitis with secondary bacterial infection. PR Newswire. July 14, 2023. Accessed July 14, 2023. https://prnmedia.prnewswire.com/news-releases/alphyn-biologics-reports-encouraging-interim-results-of-phase-2a-trial-for-atopic-dermatitis-with-secondary-bacterial-infection-301877235.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.










2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.