- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Amino Acid- Containing Supplement May Improve Clinical Efficacy of Hair Loss Treatments
Author(s):
Supplements were based on amino acids and hydrolyzed collagen and were tested in patients with androgenic alopecia and chronic telogen effluvium.
In a recent study,1 researchers found that an oral supplement containing hydrolyzed collagen and several amino acids improved the clinical efficacy of treatments being offered to patients with hair loss conditions, such as androgenic alopecia or telogen effluvium.
Bongkochrut/AdobeStock
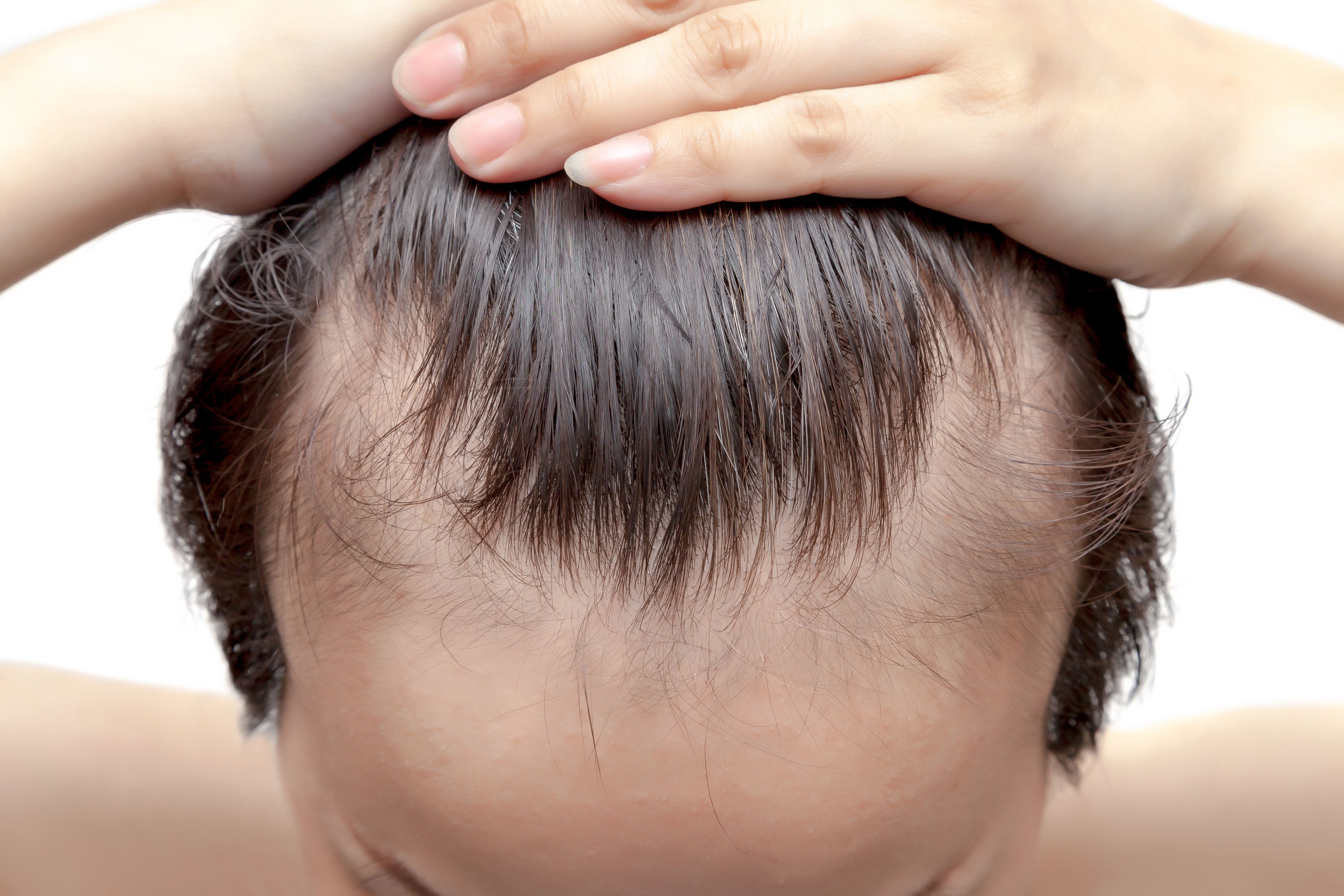
The assessor-blinded, controlled, prospective, and randomized study sought to explore the efficacy and tolerability of oral supplementation when combined with traditional therapies in patients with hair loss. Study authors Milani et al noted that adjuvant products are often used in combination with treatments due to a clinical response rate of 50% to 60% in patients receiving treatments such as finasteride and minoxidil.
They cited the correlations between amino acids and hair, noting that cysteine has been found to be an indicator of overall hair health. Additionally, taurine has been shown to promote in vitro hair survival and prevent inhibition of hair growth, with hydrolyzed collagen peptides being known to promote follicle cell proliferation and hair thickness.1
From May 2022 to November 2022, researchers enrolled 83 participants in the study, each with a clinical diagnosis of androgenic alopecia, female androgenic alopecia, or chronic telogen effluvium. All participants were required to be at least 18 years old and possess eligibility for treatment. Any patients with acute inflammatory conditions affecting their scalp, scalp conditions aside from clinical hair loss diagnoses, thyroid function alteration, iron deficiency, or relevant allergies to any ingredients in the oral supplement were excluded from participation.
The 12-week study was conducted at 6 different study centers. At the study’s baseline, all participants were evaluated and randomly divided into 1 of 2 treatment groups. One group was to receive a supplement containing hydrolyzed fish-origin collagen, taurine, cysteine, methionine, iron, and selenium. Members of 1 group (n=48) received the oral supplement to be taken once daily alongside a specific drug treatment determined by their type of hair loss while members of the other group (n=35) exclusively received specific drug treatment.
Specific drug treatments (in order of most to least frequently prescribed) used by patients in the study included topical minoxidil 2%, other (including lotions, supplementations, etc.), topical minoxidil 5%, unspecified treatments, oral minoxidil, topical finasteride 2%, and oral finasteride.
After 6 and 12 weeks, a panel of dermatologists evaluated all participants by using standardized global photographs, which were evaluated in accordance with the global assessment score (GAS). The study’s primary endpoint was GAS improvement, while secondary endpoints revolved around global clinical efficacy, global tolerability, and acceptability. In total, 76 participants completed the study in its entirety.
By week 6, researchers noted average GAS improvement only among members of the group receiving both standard treatment and the oral supplement. Both groups demonstrated GAS improvement by week 12, though researchers found the improvement to be more significant among patients receiving combination therapy. In total, 85.4% of patients receiving combination therapy (including the oral supplement in their treatment regimen) achieved GAS improvement, while this was achieved in 48.6% of patients solely receiving standard treatment. Furthermore, patients receiving combination therapy achieved higher average global clinical efficacy and tolerability.
Potential study limitations include unbalanced treatment group sizes, the study’s short duration, and the study’s lack of double blindness.
“This clinical trial demonstrated that the tested oral supplement, containing hydrolyzed fish-origin collagen, taurine, cysteine, methionine, iron and selenium was able to improve the clinical efficacy of specific anti-hair loss treatments,” according to Milani et al. “Additional studies are therefore needed to fully understand the role of nutritional supplementation in manage hair loss conditions.”
Reference
- Milani M, Colombo F. Efficacy and tolerability of an oral supplement containing amino acids, iron, selenium, and marine hydrolyzed collagen in subjects with hair loss (androgenetic alopecia, AGA or Faga or telogen effluvium). A prospective, randomized, 3‐month, controlled, assessor‐blinded study. Skin Res Technol. 2023;29(6). doi:10.1111/srt.13381
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














