- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
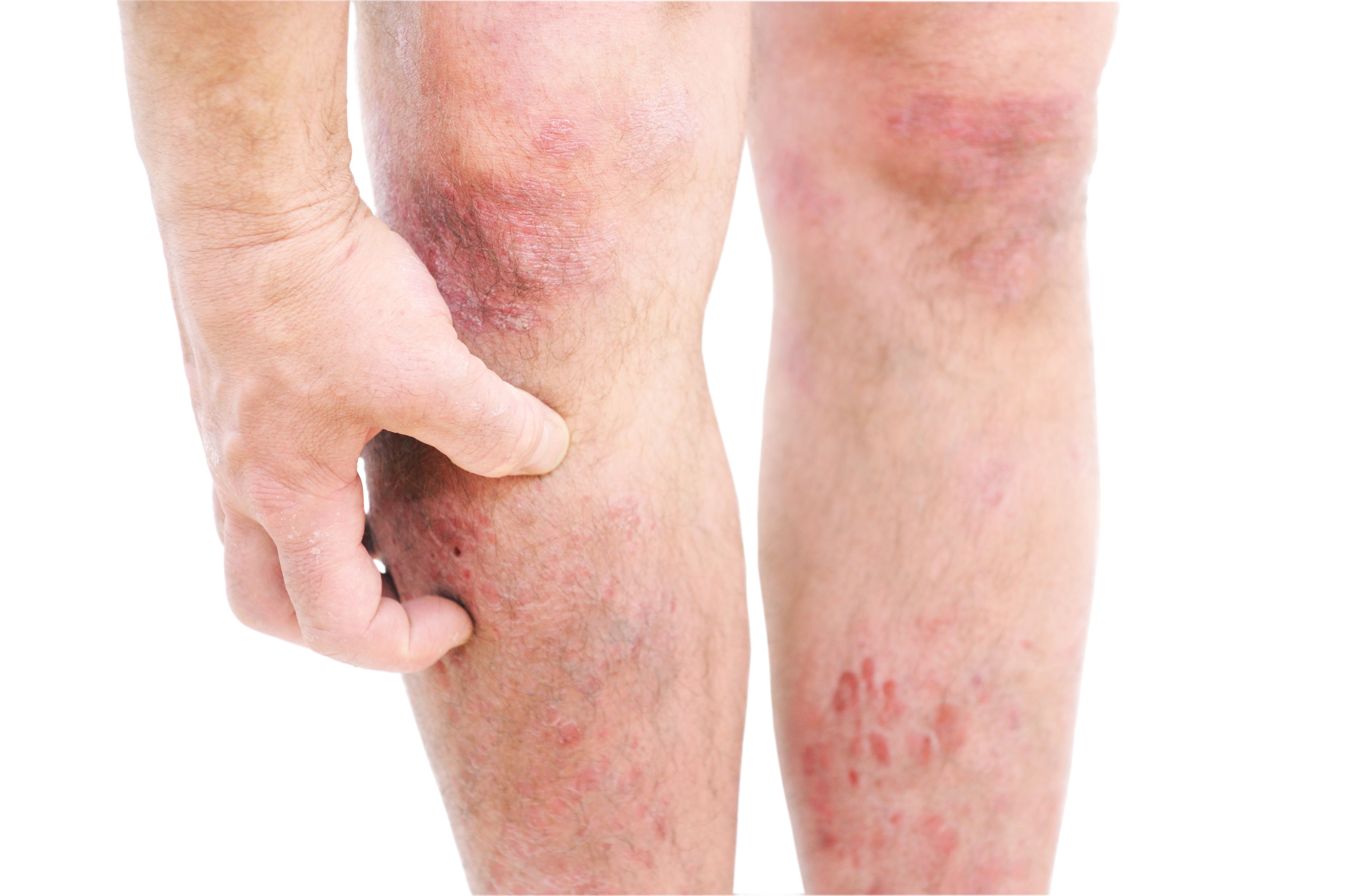
Amlitelimab Demonstrates Sustained Improvements in Atopic Dermatitis Signs and Symptoms
Author(s):
Late-breaking data demonstrating the sustained improvements was presented at the American Academy of Dermatology Annual Meeting.
Late-breaking data recently presented at the American Academy of Dermatology Annual highlighted sustained off-drug improvements of atopic dermatitis (AD) signs and symptoms with amlitelimab for 28 weeks, supporting its role as a potential best-in-class maintenance therapy.1
At the conference, positive results from part 2 of the investigational amlitelimab phase 2b study STREAM-AD (NCT05131477)2 were shared, revealing sustained improvement of signs and symptoms for 28 weeks in adults with moderate to severe AD who had previously responded to amlitelimab and continued treatment. These findings shed light on the durability of response, supporting the evaluation of a less frequent dosing regimen.
Amlitelimab is a fully human non-T cell depleting monoclonal antibody which targets OX40-Ligand, a key immune regulator, with the aim of restoring balance between pro-inflammatory and regulatory T cells. Its efficacy and safety are currently under clinical investigation, with phase 3 pivotal trials underway for AD and phase 2 studies in progress for other indications.
The STREAM-AD phase 2b study enrolled 390 adult patients with moderate-to-severe AD across multiple countries. It was comprised of 2 parts, with part 1 focusing on a 24-week treatment period and part 2 exploring a 28-week maintenance/withdrawal phase.
“It’s unprecedented to see this type of durability of clinical response, which we believe could be very meaningful to patients and is the reason why we selected an every 12-week dosing regimen in the AD pivotal program," said Naimish Patel, MD, head of Global Development, Immunology and Inflammation at Sanofi, in a news release.1
"AD is a chronic, lifelong disease, which means we must strive to provide a portfolio of solutions to patients that matches their individual needs and puts as little burden on them as possible," Patel said. "We are also moving with speed in our exploration of amlitelimab's potential in 5 other chronic inflammatory diseases, including asthma, hidradenitis suppurativa, scleroderma, celiac disease, and alopecia. In addition, we are exploring 6 other innovative MOAs in 8 dermatologic indications underscoring our commitment to patients with high unmet medical needs.”
In the study's second part, responders to amlitelimab during the initial 24-week treatment period were re-randomized to explore the maintenance of clinical response. Those who continued amlitelimab treatment showed sustained high responder rates, as did participants who were taken off treatment. Amlitelimab was well-tolerated, with no new safety concerns identified, reinforcing its potential as a safe and effective treatment option for AD.
“Despite available treatment options, not all patients with moderate-to-severe atopic dermatitis respond sufficiently to these treatments, and many continue to suffer from skin lesions and symptoms such as persistent itch, which can have a high impact on their day-to-day lives," said Stephan Weidinger, MD, PhD, the director, professor, chair of the Department of Dermatology and Allergy at University Hospital Schleswig-Holstein.1 "Results from this part of the study indicate amlitelimab’s potential for durable off-drug efficacy which supports the evaluation of a less frequent every 12-week dosing. This could offer an important benefit in the treatment of AD patients.”
References
- New phase 2b results for amlitelimab support potential for best-in-class maintenance of response in atopic dermatitis. News release. Sanofi. March 11, 2024. Accessed March 20, 2024. https://www.sanofi.com/en/media-room/press-releases/2024/2024-03-11-06-00-00-2843456
- Study testing response effect of KY1005 against moderate-to-severe atopic dermatitis, the STREAM-AD study (STREAM-AD). ClinicalTrials.Gov. Accessed March 20, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05131477
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.