- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Amlitelimab Significantly Improves Average EASI Scores in Phase 2b AD Study
Author(s):
Sanofi’s phase 3 program for amlitelimab in atopic dermatitis will start in early 2024.
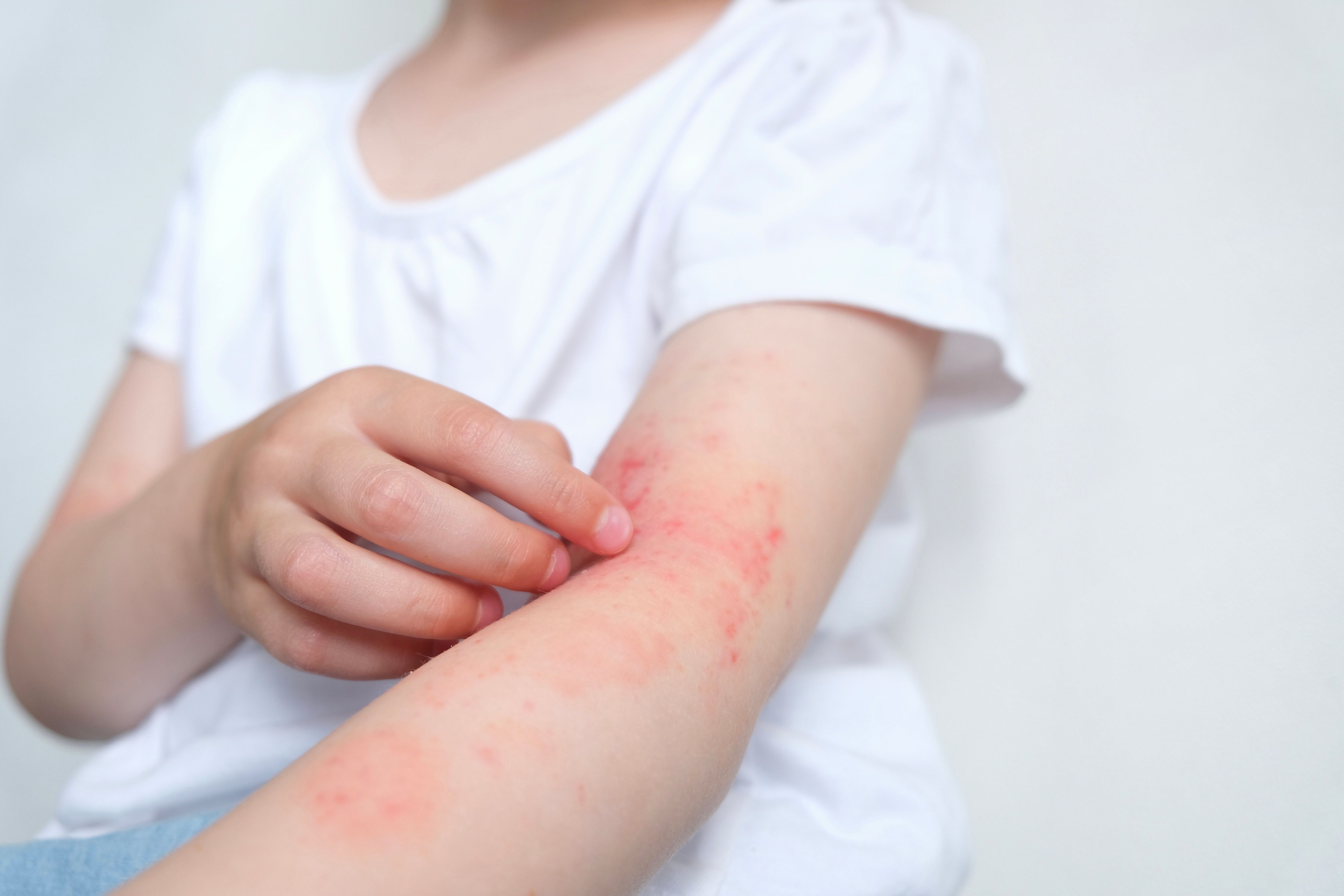
Sanofi announced today positive data from its phase 2b study, STREAM-AD (NCT05131477), of amlitelimab significantly improving signs and symptoms of moderate to severe atopic dermatitis (AD) in adults whose AD cannot be managed with topical medications or for whom topical medications are not recommended. The late-breaking data was presented at the European Academy of Dermatology and Venereology (EADV) 2023 Congress in Berlin, Germany. Based on these results, Sanofi’s phase 3 program for amlitelimab in atopic dermatitis will start in early 2024.1
Key findings from the study include:
- Subcutaneous treatment with amlitelimab demonstrated statistically significant improvements in the primary end point of percent change in Eczema Area and Severity Index (EASI) score from baseline at 16 weeks compared to placebo for all 4 doses evaluated
- Patients treated with amlitelimab 250 mg Q4W with 500 mg loading dose (LD) had the numerically highest response versus placebo, with a 61.5% reduction in EASI from baseline at week 16 (P<0.0001) and a 64.4% reduction at week 24 (P<0.0001) vs. 29.4% at week 16 and 27.6% at week 24 for placebo
- Across all doses, clinically meaningful and nominally significant improvements were observed in all key secondary end points at weeks 16 and 24, including Investigator Global Assessment response of 0 (clear) or 1 (almost clear skin) (IGA 0/1), 75% reduction from baseline in EASI (EASI-75) and weekly average reduction of Peak Pruritus Numerical Rating Scale ≥4 points from baseline, except the 250 mg (no LD) in IGA 0/1 at week 16 (p=0.0562)
- 22.1% and 45.5% of patients treated with amlitelimab 250 mg with LD achieved IGA 0/1 at weeks 16 and 24, respectively, compared to 5.1% and 11.4% of placebo patients
- 40.3% and 54.5% of patients treated with amlitelimab 250 mg with LD achieved EASI-75 at weeks 16 and 24, respectively, versus 11.4% and 17.7% on placebo
- Across all doses at weeks 16 and 24, amlitelimab significantly reduced levels of biomarkers elevated in AD, including Th2-related IL-13 and TARC, Th17/Th22-related IL-17A and IL-22, and blood eosinophil counts, with significant reduction seen as early as week 4 in the 250 mg with LD arm.
Overall, amlitelimab was well-tolerated across all dose arms and no new safety signals were reported. The total rates of treatment-emergent adverse events (TEAEs) were 67.4% for amlitelimab and 60.3% for placebo. TEAEs more commonly associated with amlitelimab compared to placebo were nasopharyngitis (11.0% amlitelimab, 9.0% placebo), COVID-19 (7.7% amlitelimab, 6.4% placebo) and headache (6.1% amlitelimab, 2.6% placebo). In the placebo group (38.5%), the worsening of AD was more common compared to amlitelimab (17.1%). No adverse events such as fever or chills, oral ulcers, or imbalances with conjunctivitis were reported.
“The data presented at EADV provide more detailed insight into amlitelimab’s potential as a best-in-class therapy for people with atopic dermatitis. In addition, our ability to pursue a differentiated dosing regimen could be very meaningful to patients. We look forward to initiating a larger Phase 3 development program for amlitelimab in atopic dermatitis in the first half of 2024, which further underscores our commitment to delivering a diverse range of solutions for this chronic condition,” said Naimish Patel, MD, the head of global development for immunology and inflammation at Sanofi, in the news release.
Amlitelimab is a fully human non-depleting monoclonal antibody that binds to OX40-Ligand, and according to Sanofi, has the potential to be a first-in-class treatment for a range of immune-mediated diseases and inflammatory disorders such as moderate to severe AD and asthma. Amlitelimab may be able to restore balance between pro-inflammatory and regulatory T cells by targeting OX40-Ligand.
Reference
- Late-breaking amlitelimab phase 2b data presented at EADV show potential best-in-class profile in atopic dermatitis. Sanofi. News release. October 13, 2023. Accessed October 13, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-10-13-14-00-00-2760021
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.