- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Approval process straightforard, officials say
Author(s):
National report - The Food and Drug Administration's approval process can seem mysterious to most physicians, who lack the time to study the process, experts say.

"They assume it's this big, cumbersome process. And in fact it isn't, if one takes a look and understands why it is as it is," says Ella Toombs, M.D., a former FDA dermatology medical officer who is now assistant professor of dermatology, Rush University Medical Center, and director of Aesthetic Dermatology of Dupont Circle, Washington, D.C.
The 1938 Food, Drug and Cosmetic Act gives the FDA authority to regulate drugs, biologics and devices. The agency regulates products by their intended uses, says Freddie Ann Hoffman, M.D., who worked for the FDA in various capacities from 1985 to 1989.

Approval process for drugs
The regulatory process for drugs begins with a pre-Investigational New Drug meeting (IND) and continues even after formal approval - "a total lifecycle approach" requiring ongoing reports and postmarketing surveillance, says Susan Walker, M.D., director, FDA Division of Dermatology and Dental Products.
For manufacturers, the process begins with sifting through perhaps thousands of prospects to discover a promising candidate. Then researchers conduct extensive preclinical testing of the substance's absorption, distribution, metabolism and excretion characteristics.
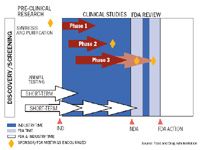
At this stage, "One wants FDA or other agencies to say, 'Yes, we like your plan,' or 'Change this or that,'" says Alberto Grignolo, general manager of Parexel Consulting, a subsidiary of Parexel International that helps companies through the drug approval process.
Clinical trials
The IND contains the pre-clinical data and proposed plans for study in a human population.
After a company submits an IND, "The center has 30 days to review the information and make a decision whether to permit the sponsor to begin human trials," Dr. Walker says.
The process then moves into three phases of clinical trials:
Some of the most important meetings occur after sponsors have collected phase 2 data and require FDA help in planning phase 3 testing, Dr. Walker says. Products that pass phase 3 scrutiny proceed to New Drug Applications (NDAs).
"We have 10 months to review NDAs, and we have a very good success rate" meeting this deadline, Dr. Walker says.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











