- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Arcutis Enrolls Last Participant in ARQ-255 Trial for AA
Author(s):
Arcutis' study of ARQ-255 aims to address the lack of FDA-approved topical treatments for alopecia areata, a condition affecting roughly 1 in 500 people.
Image Credit: © Alex Papp - stock.adobe.com
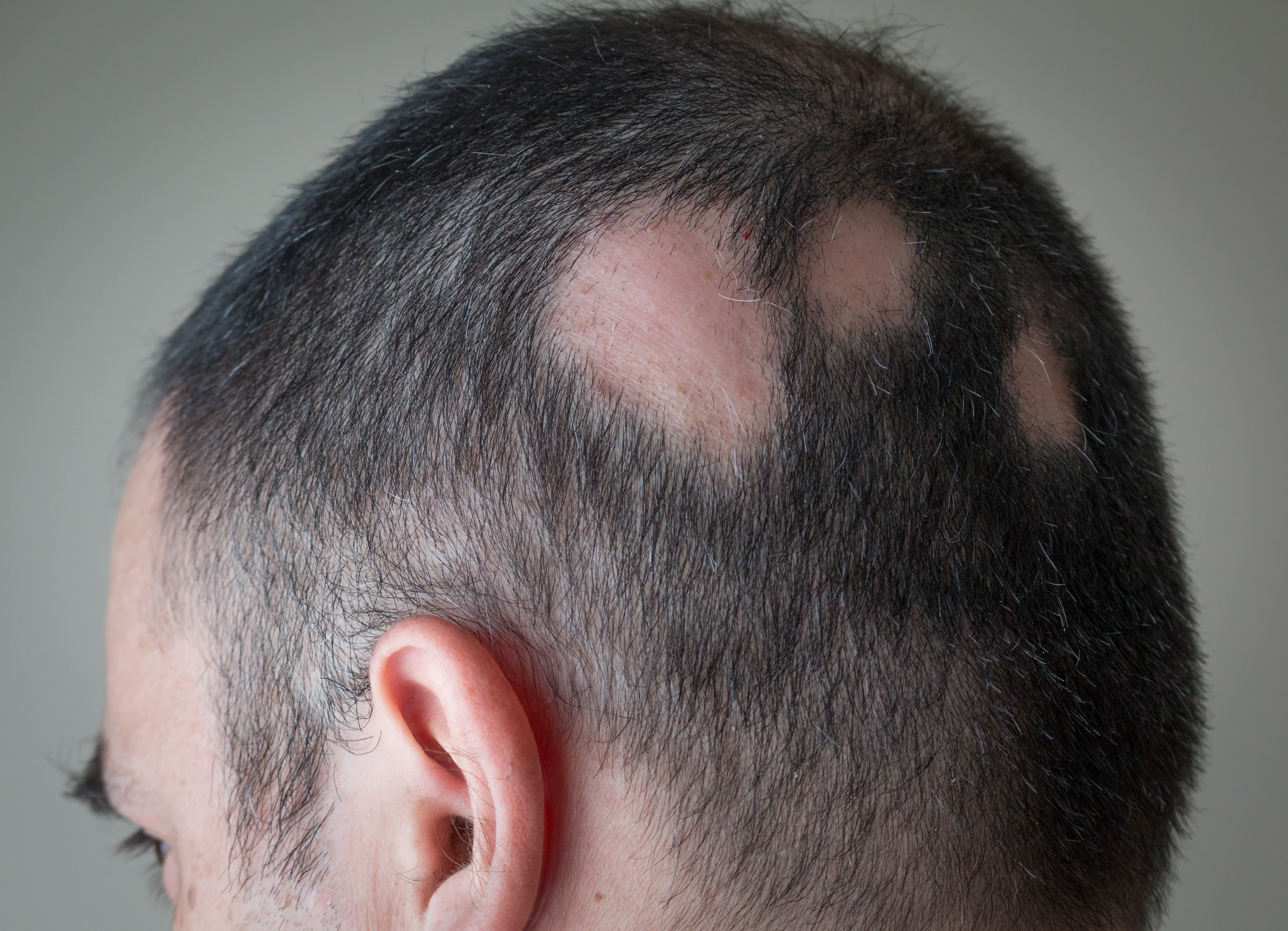
Arcutis Biotherapeutics announced today the enrollment of the last participant in the phase 1b study for ARQ-255, a novel treatment for alopecia areata (AA). This study marks a step forward in addressing a condition for which no FDA-approved topical treatments currently exist.1
According to a press release from the company, ARQ-255 is a topical suspension containing ivarmacitinib, a highly selective janus kinase 1 (JAK1) inhibitor. The formulation leverages Arcutis' proprietary 4D technology to penetrate deeply into the skin, targeting the base of the hair follicle, where inflammation occurs in AA. AA is an autoimmune disorder characterized by sudden, patchy hair loss on the scalp, face, and other areas of the body.
“Alopecia areata affects approximately 1 in 500 people, yet today there are no FDA approved topical treatments for this devastating condition,” said Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis. “The safety, tolerability, and pharmacokinetics data generated from this first-in-human study will provide valuable information to inform our ARQ-255 clinical development program and advance our vision of bringing innovation to the treatment of immune-mediated skin conditions where there has been little advancement in decades.”
The phase 1b study is a vehicle-controlled, double-blind, multicenter trial that aims to assess the safety, tolerability, pharmacodynamics, and pharmacokinetics of ARQ-255 in both healthy volunteers and individuals with patchy AA. The study involves 44 participants and Arcutis expects to report results in the first half of 2025.
When the first participant was enrolled in the study in December 2022, Frank Watanabe, president and CEO at Arcutis, had this to say: “Alopecia areata is an immune condition that not only causes hair loss, but also causes significant negative impact on an individual’s emotional and mental wellbeing. Today, there are no FDA-approved topical therapies to treat the condition. We are delighted to take this first step in the clinical development of ARQ-255, which leverages our unique 4D technology to deliver drug to the site of inflammation deep in the hair follicle.”2
Arcutis noted that AA presents significant challenges for topical treatments due to the deep-seated inflammation affecting the hair follicles. The company stated ARQ-255's formulation is designed to overcome these challenges by delivering the drug effectively to the site of inflammation, offering potential new hope for patients who currently have limited options.
The company stated it has a strong focus on developing therapies for immune-mediated dermatological conditions. Its portfolio includes 3 FDA-approved products and a “robust pipeline” of clinical programs targeting various inflammatory skin conditions such as scalp and body psoriasis, atopic dermatitis, and AA.
References
- Arcutis completes enrollment of phase 1b alopecia areata study evaluating ARQ-255. News Release. Arcutis Biotherapeutics. September 5, 2024. Accessed September 5, 2024. https://investors.arcutis.com/news-releases/news-release-details/arcutis-completes-enrollment-phase-1b-alopecia-areata-study
- Arcutis enrolls first patient in phase 1b alopecia areata study evaluating ARQ-255. News Release. Arcutis Biotherapeutics. December 5, 2022. Accessed September 5, 2024. https://www.arcutis.com/arcutis-enrolls-first-patient-in-phase-1b-alopecia-areata-study-evaluating-arq-255/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











