- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Autologous Cell Therapy Effective in Wound Treatment
Author(s):
Researchers applied EB-101, a new cell therapy, to patients with recessive dystrophic epidermolysis bullosa with positive results.
Investigators assessed the efficacy of EB-101, an investigational autologous cell therapy, on patients with recessive dystrophic epidermolysis bullosa (RDEB) and found that the majority of wounds (81%) treated with EB-101 showed healing of ≥50% and a reduction in pain severity, meeting the co-primary endpoints.1 The findings were reported in a poster presented at the 2023 Society for Pediatric Dermatology Meeting, July 13-16, 2023, in Asheville, North Carolina.
tibanna79/AdobeStock
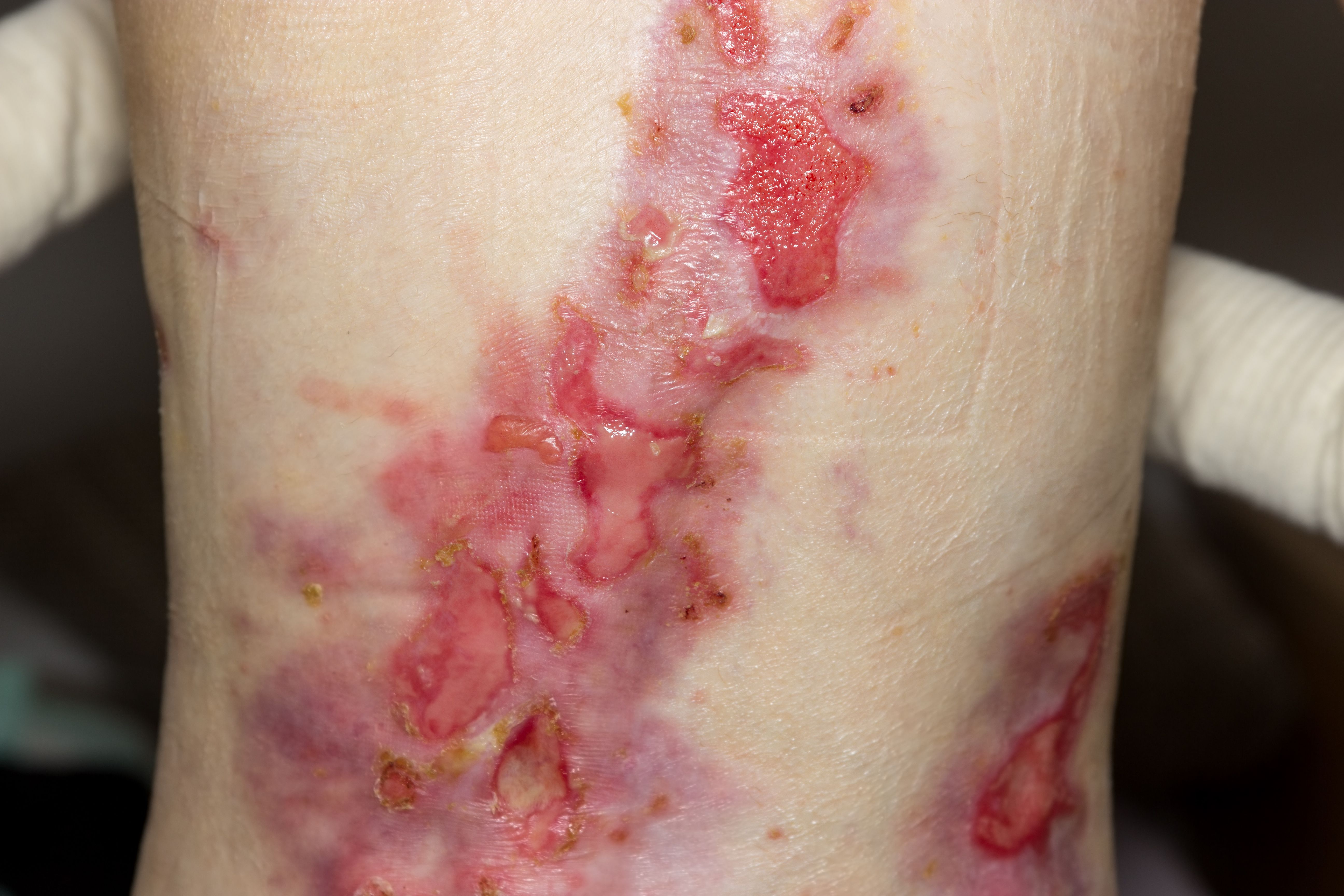
RDEB patients “have mutations in COL7A1, the gene encoding Type VII collagen.”1 By taking 2 punch biopsies from a patient with RDEB, 6 grafts measuring 40 cm2 are produced in 25 days using a “retrovirus expressing the corrected COL7A1 gene.”1 In a phase 3, randomized, intrapatient-controlled trial (VIITAL), 11 RDEB patients with large, chronic, locational matched wound pairs received EB-101 treatment or standard of care (controls).
Eligible participants had to be at least 6 years old, have 2 or more matched, large chronic wounds, and have no evidence or history of squamous cell carcinoma in the area that would be treated with EB-101. Co-primary endpoints were ≥50% wound healing at week 24, and a reduction in pain at week 24. A secondary endpoint was complete wound healing of the wound at weeks 12 and 24.
EB-101 was applied to 43 wounds across 11 patients, and control treatment was used across 43 matched wounds. At week 24, 81% of wounds treated with EB-101 achieved ≥50% healing compared to 16% of control wounds (P < .001). Severity of pain from baseline to week 24 was reduced by 3.07 for EB-101—treated wounds compared to 0.90 for control wounds (P < .001).
EB-101 was also effective in ≥75% and 100% wound healing, meeting secondary endpoints. Additional exploratory endpoints that were met included a reduction in itch and wound blistering. A decrease in pain at earlier weeks (6 or 12) was also achieved.
Based on their study, researchers concluded that, “EB-101 application demonstrated a favorable risk-benefit profile in both adult and pediatric patients with RDEB.”
Reference
- Mittal V, Tang J, Marinkovich M, et al. Phase 3 randomized, intrapatient-controlled trial of an investigational collagen type VII gene-corrected autologous cell therapy, EB-101, for the treatment of RDEB. Poster presented at the 2023 Society for Pediatric Dermatology Meeting; July 13-16, 2023. Asheville, NC.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.