- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Berdamizer Gel Safe, Well-Tolerated in Patients With Molluscum Contagiosum
Author(s):
The treatment was deemed effective following a phase 3 study.
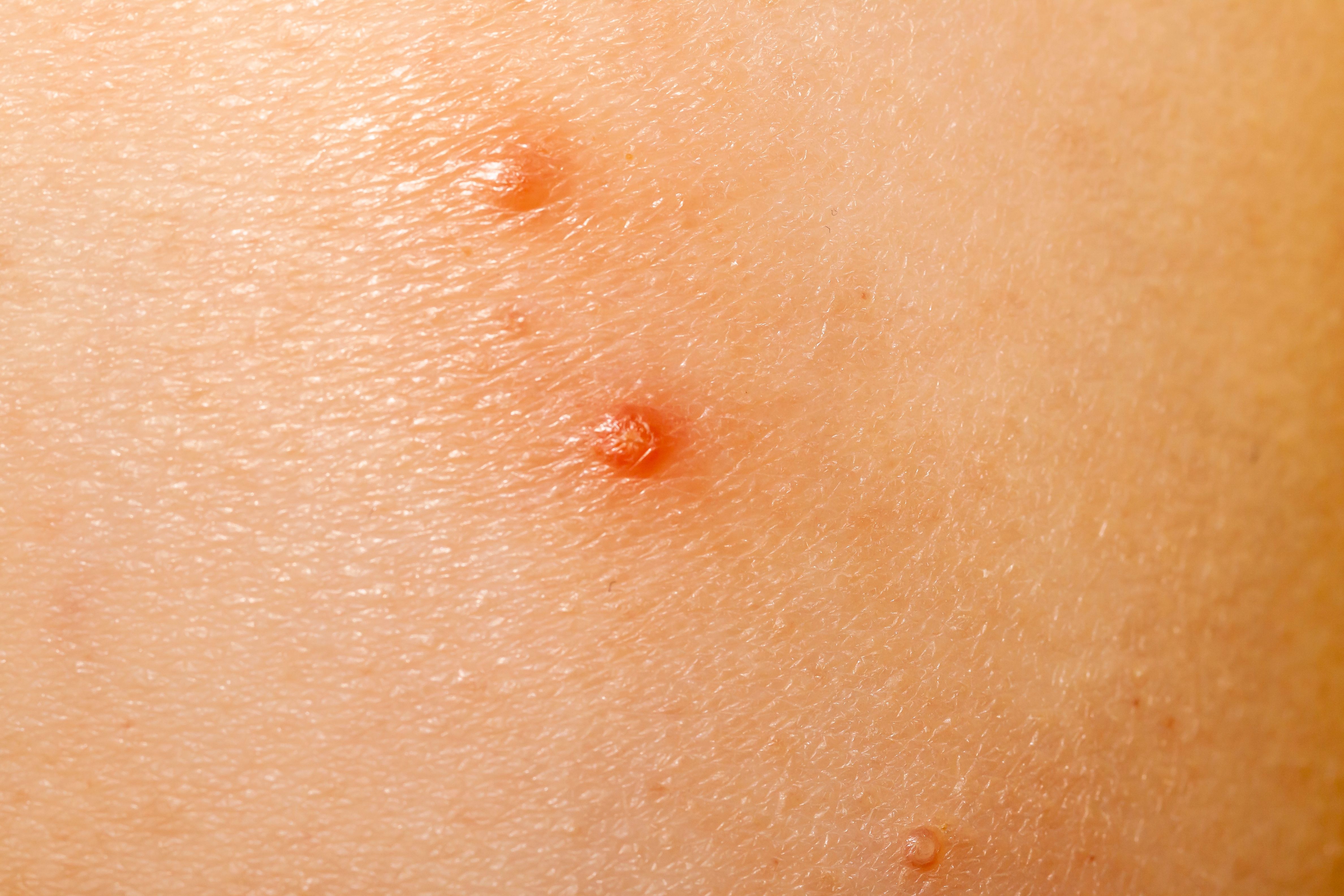
Berdamizer gel 10.3% is both safe and effective in treating molluscum contagiosum, according to a poster presentation from the American Academy of Dermatology (AAD) Annual Meeting.1
Lukassek/AdobeStock
In January, Novan Inc. submitted an NDA to the US Food and Drug Administration (FDA). The filing was based on the conclusion of a phase 3 study. In early March, the FDA accepted Novan’s filing, citing a Prescription Drug User Fee Act goal date of January 5, 2024.
The B-SIMPLE1, 2, and 4 trials were randomized and double-blind, spanning several locations and including both a berdamizer gel treatment and a vehicle-controlled treatment. Participants (n=1598) were patients with molluscum contagiosum greater than 6 months of age with a range of 3-70 overall lesions at the start of the trials.
Following participant selection, researchers separated participants into treatment groups. A total of 917 individuals were assigned berdamizer gel as treatment, and 681 were assigned the vehicle gel. Upon randomization and assignment, study participants were asked to apply either the berdamizer gel or the vehicle gel on molluscum contagiosum lesions for a period of 12 weeks.
In order to determine berdamizer gel’s safety and efficacy, researchers sought the following endpoints:
- PRIMARY ENDPOINT: Proportion of patients with complete clearance of all treatable MC lesions at week 12
- SECONDARY ENDPOINT: Proportion of patients with complete clearance of all treatable MC lesions at week 8
- SAFETY MEASURE/ENDPOINT: Treatment-emergent adverse events (TEAE) and local skin reactions (LSR) through week 24
All participants were assessed for LSR and scored on an LSR composite scale.
The study found that TEAEs related to the study drug were mostly mild or moderate in nature, leading to discontinuation in a small number of participants (n=46), the majority of which had been using berdamizer gel. Common TEAEs included application site pain, redness, pruritus, exfoliation, and dermatitis.
Following the 12-week period, researchers conducted an additional 12-week period of safety follow-ups.
According to the study authors, while there is currently no FDA-approved treatment for molluscum contagiosum, “berdamizer gel 10.3% addresses many of the challenges of [nitric oxide] NO delivery.”
“The B-SIMPLE phase 3 program demonstrates favorable safety and tolerability of berdamizer gel 10.3%,” study authors wrote.
Reference
- Browning J, Hebert A, Paller A, et al. Safety of berdamizer gel 10.3% in the treatment of molluscum contagiosum: Integrated results from the B-SIMPLE phase 3 program. Presented at the 2023 American Academy of Dermatology Annual Meeting; March 17-21, 2023; New Orleans, LA. Accessed March 17, 2023. https://aad-eposters.s3.amazonaws.com/AM2023/poster/42087/Safety+of+Berdazimer+Gel+103+in+the+Treatment+of+Molluscum+Contagiosum+Integrated+Results+from+the+B-SIMPLE+Phase+3+Program.pdf






