- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Better outcomes: Topical agents may enhance surgical results
Topical agents in combination with surgery may lead to better results. Pre-op treatment can make lesions more visible, define margins and reduce tumor size. Post-op use can reduce the amount of residual cancer.

Key Points

Dr. Spencer has experimented with topical agents before surgery to enhance the visibility of lesions and after surgery to reduce recurrence rates.
Based on his own experiments - case studies of individual patients and a small clinical trial - he urges dermatologists to think pluralistically when it comes to the treatment of some skin conditions.
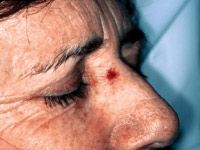
Pre-operative use
"Extramammary Paget's Disease (EMPD) is a rare tumor, usually in the groin area. It can be very hard to see where the lesions start and stop," Dr. Spencer says.
Poor visibility leads to poor results. Following wide excision, the recurrence rate is up to 40 percent. Dr. Spencer uses topical 5-FU cream on the lesions prior to surgery. This causes inflammation, making the lesions redder than surrounding tissue and bringing definition to the margins.
Visibility is also impaired when a patient presents with squamous cell carcinoma (SCC) situated within and obscured by a sea of actinic keratoses (AK). This typically manifests on the back of the hand or on the scalp. By treating the AK first with 5-FU or imiquimod, the AK lesions will recede, and the carcinoma will pop out visually.
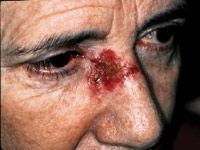
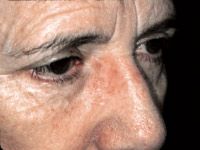
Post-operative use
The most common treatment for BCC is curettage and electrodessication (C&D). Recurrence rates vary by size, histology and location. Even when the lesion is uncomplicated, less than 1 cm in size, and in a low-risk location, histological samples taken after C&D show residual BCC in 21 percent to 37 percent of tissues.
However, at five years post-op, the actual recurrence rate is only 5 percent to 10 percent.
These facts are well documented, Dr. Spencer says. The question is, why is there a large difference between the short-term evidence of BCC and the long-term recurrence rate? Dr. Spencer suggests that inflammation may be one of the factors contributing to the healing process.
To test the hypothesis, he devised a study in 2004, enrolling 20 patents with primary nodular BCC. All patients were treated with three cycles of C&D. Following surgery, 10 patients received topical imiquimod nightly for a month, and 10 patients received placebo.
Two months after the surgical end point, he excised the tumor area and step sectioned tissue. Results published in 2006 indicated a 10 percent rate of residual tumor in the group receiving treatment with imiquimod versus 40 percent in the control group. Dr. Spencer concluded that application of topical imiquimod following C&D results in a significantly decreased frequency of residual tumor.
In contrast to other studies that have noted inferior cosmetic results with imiquimod, Dr. Spencer found that the majority of lesions in the subject group were flat and slightly pink, while the majority of control were atrophic and hypopigmented. In the final analysis, use of imiquimod after C&D for treatment of nodular BCC had two benefits - less residual cancer and better cosmetic appearance. However, it had one drawback - delayed healing. At four weeks, the entire control group but only half of the subject group had healed.
"We know that, when doing C&D, we'll leave more cancer behind unless treated with imiquimod," Dr. Spencer says.
"That's significant, because it suggests you can use medicine to improve surgical results in tricky areas or when dealing with tough tumors."
He speculates that topical imiquimod might improve surgical results in high-risk BCC and SCC, lentigo maligna, EMPD and keloids, but says more research and larger clinical trials are needed.
Disclosures: 3M Pharmaceuticals, maker of imiquimod, funded Dr. Spencer's research as well as the study by Dr. Torres. Dr. Spencer is also a speaker and consultant for Graceway Pharmaceuticals, which markets imiquimod under the trade name Aldara.





