- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Bioelectric dressing treats acute and chronic wounds
The use of bioelectric stimulation is key to a novel wound-healing technology that continues to be explored in studies, says a dermatologist who has investigated the clinical technology.

Key Points

Known by the trade name Procellera (Vomaris), the bioelectric dressing is approved by the Food and Drug Administration to treat partial and full-thickness wounds such as pressure ulcers, venous ulcers, diabetic ulcers, burns, surgical incisions and graft sites. When moistened, the dressing is activated and generates a sustained electric stimulation to the wound. The wound is covered with an overlying dressing to keep the wound moist and the dressing active.
"Bioelectric woundcare actually stimulates wounds to heal," explains Scott Sheftel, M.D., F.A.A.D., associate professor at the University of Arizona, Tucson, Ariz., and principal dermatologist with Sheftel Associates Dermatology. "That is the underlying premise of it."
"We have been working with a self-contained dressing that behaves like a battery when in contact with a wound," he explains. "When moistened, it generates one volt of sustained current to the wound surface."
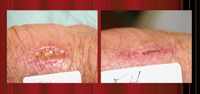
"They exchange electrons and that establishes the current, just like a battery would," Dr. Sheftel says. "Normally, the skin has physiological electrical activity that is measurable. When there is trauma, there is a gradient of electrical activity within the wound. This dressing mimics the normal physiologic response to healing."
The dressing influences wound healing through the current that it generates, ranging from two to 10 microamperes on the surface of the device. In addition, it has antimicrobial properties, in that it destabilizes bacterial and viral cell walls. The electrical stimulation also has analgesic properties in that it targets pain receptors.
"It is gaining acceptance as an alternative to traditional woundcare," Dr. Sheftel says.
Accelerated healing
The therapy has hastened healing in acute wounds such as lacerations and surgical incisions, as well as chronic wounds such as diabetic ulcers and stasis ulcers, according to Dr. Sheftel.
"Some chronic wounds can be traditionally very difficult to heal, and they have failed wound vac (vacuum-assisted closure) and hyperbaric therapy, and we have obtained good results (with bioelectric dressings)," Dr. Sheftel says.
In case reports looking at the impact of Procellera on a diabetic foot ulcer, a venous leg ulcer and a pressure ulcer, clinicians observed improvements in wounds, to the point of full closure as well as a decline in pain and inflammation.
Patients are not receiving episodic care with Procellera as they are with wound vacuum-assisted closure and hyperbaric therapy. Instead, they are receiving ongoing therapy via the dressing.
"It is constant stimulation to the site of the wound," Dr. Sheftel says.
Dr. Sheftel and colleagues have treated hundreds of patients with Procellera and submitted research to peer-reviewed journals that suggests increased efficacy compared with other commercial therapies.
Procellera research
At present, there are ongoing randomized, controlled, clinical trials to demonstrate the efficacy of Procellera, yet its safety beyond 28 days of use has not been studied.
"We have been able to statistically show superior healing," Dr. Sheftel says. "We can heal wounds faster and improve patient comfort."
In some instances, Procellera has been used in combination with approaches such as negative pressure wound therapy to optimize the outcome of a wound.
A three-arm pilot study compared Procellera with a self-adhesive bandage and a silicone foam island dressing in treating wounds that resulted after curettage and electrodessication of small squamous cell and basal cell skin cancers. Photos were taken of wounds, and Dr. Sheftel and colleagues recorded any adverse events, such as infections, erythema or edema. Patients noted discomfort through a numeric pain intensity scale.
At three weeks, investigators observed an 88 percent decrease in wound size for patients treated with Procellera, a 74 percent reduction in wound size for patients treated with the foam island dressing, and a 77 percent reduction in wound size for patients treated with the adhesive bandage. The Procellera-treated patients noted increased wound comfort.
Patients who are allergic or sensitive to silver, zinc and/or polyester should avoid using Procellera. In addition, patients undergoing magnetic resonance imaging, electrocardiogram or electroencephalogram should avoid using Procellera.
Disclosures: Dr. Sheftel is a consultant for Vomaris, the manufacturer of Procellera.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











