- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Biosimilars Offer VA Cost Savings in Psoriasis Treatment
Author(s):
Enhanced education can reduce uncertainty among both patients and providers, with pharmacists playing a key role as primary sources of patient information.
Image Credit: © Daniel Beckemeier - stock.adobe.com
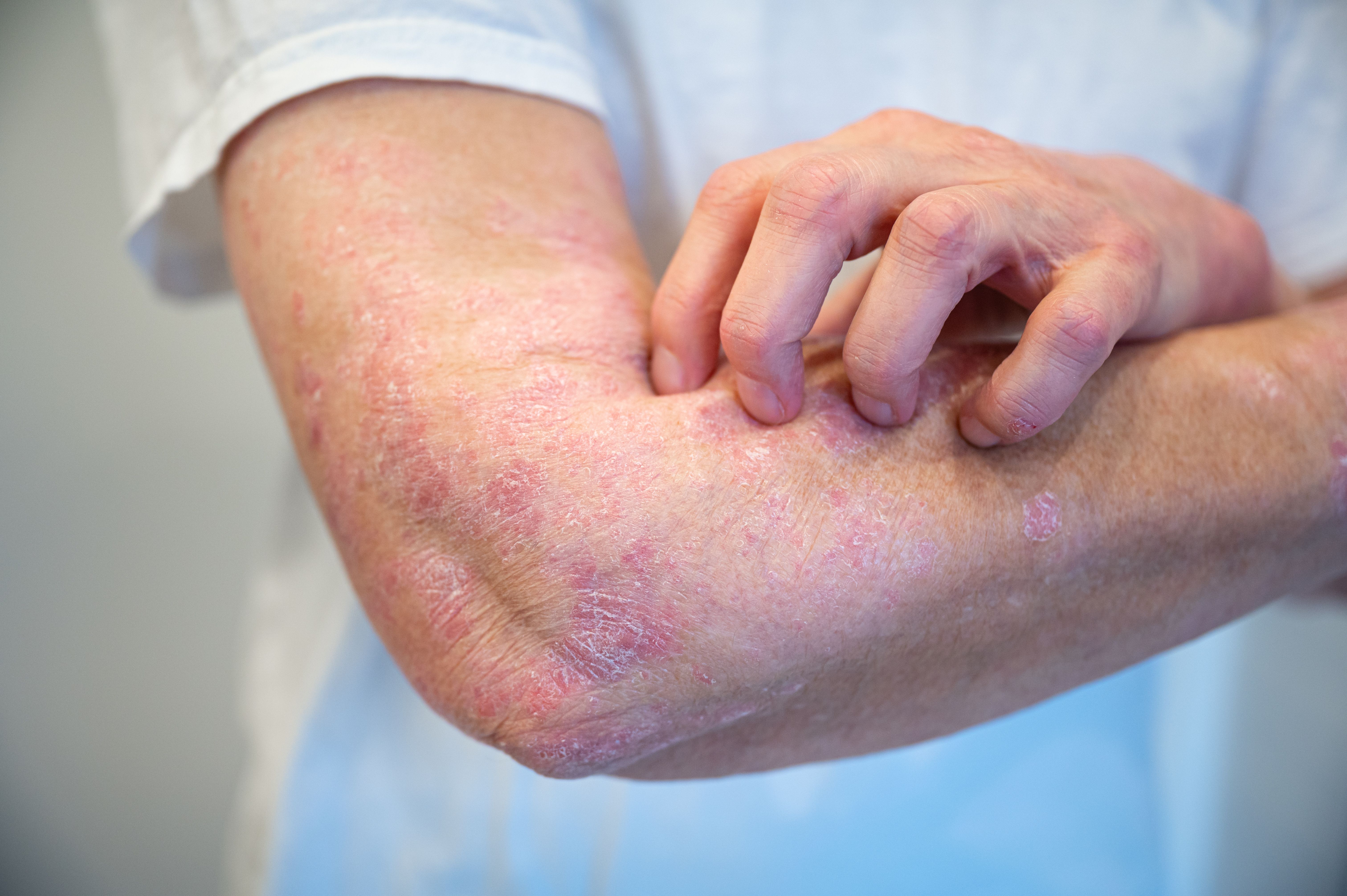
In a review article published in the Journal of Dermatological Treatment, biosimilars showed an overall effective, safe, and well-tolerated profile for patients who have psoriasis and are being provided cost savings through Veteran Affairs. However, investigators said additional education is needed to address “sources of ambivalence for both patients and providers to assist in further uptake of biosimilars for treatment of psoriasis.”1
According to the National Psoriasis Foundation, over 8 million Americans have a form of psoriasis and an estimated 30% of individuals also develop psoriatic arthritis. Patients with moderate-to-severe disease have expressed greater negative impacts on their quality of life and also experience higher rates of comorbidities, including cardiovascular disease, heart attack, stroke, metabolic syndrome, and depression.2
In mild cases of disease, topical therapies, such as corticosteroids, vitamin D analogues, and calcineurin inhibitors can control symptoms and flare-ups. For moderate-to-severe cases, systemic therapies, including oral medications, phototherapy, or biologics could be used. Biosimilars can be used as substitution of approved biologic drugs since they have comparable efficacy, safety, and immunogenicity. They have been known to be more cost-effective compared with the reference product, enhancing accessibility for patients that need it.3
In the review, articles were pulled and evaluated from PubMed, Wright State University Libraries, and Google Scholar using key words such as “biosimilars for psoriasis,” “switching studies,” and “cost savings.” Overall, investigators found that biosimilars showed near-equivalent effectiveness compared with the reference products, in medical and non-medical switches, as well as in batch-to-batch analyses. There were also similar drug survival and immunogenicity findings, according to the authors, but the factors improved when used as the first systemic treatment or as a non-medical switch. For safety, investigators also noted that biosimilars and reference products had similar rates of adverse effects, infections, and infusion reactions.1
Even with the growing evidence, the study authors reported concerns from providers on biosimilar usage for psoriasis. In an online survey, 63% of physicians said they felt like there is not enough information to be comfortable to prescribe biosimilars and 44% were confident of the safety of biosimilars. One of the factors of ambivalence is the “threat of mandatory prescriptions,” which would potentially limit the choice of the prescriber to biosimilars, according to the study authors.1
Another issue with uptake was the compliance of biosimilars, which show that there are higher discontinuations in switching studies from the originator to the biosimilar. For example, in one study, patients from the Veteran’s Health Administration showed that those with chronic inflammatory diseases were between 2.88 to 3.38 times more likely to discontinue therapy than those who remained on the reference product.1
Between provider ambivalence and patient compliance of biosimilars, the study authors stated that additional education is needed for both providers and patients about the use of biosimilars for psoriasis. Pharmacists are a vital source of information about biosimilars, especially for patients with psoriasis.1
References
- Reese R, Nanavath SR, Martin J, Travers JB, Rohan CA. A review of biosimilars in psoriasis: impacts on efficacy, safety, access, and a first-hand look at biosimilar cost savings within the department of veterans affairs. J Dermatolog Treat. 2024;35(1):2402912. doi:10.1080/09546634.2024.2402912
- National Psoriasis Foundation. Psoriasis Statistics. December 21, 2022. Accessed September 20, 2024. https://www.psoriasis.org/psoriasis-statistics/
- Fero A. Biosimilars Play Growing Role in Psoriasis Treatment Strategies. Pharmacy Times. May 13, 2024. Accessed September 20, 2024. https://www.pharmacytimes.com/view/biosimilars-play-growing-role-in-psoriasis-treatment-strategies
[This article was originally published by our sister publication, Pharmacy Times.]
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







