- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Breaking Down Benzene Testing Methods of Benzoyl Peroxide Products
Author(s):
On March 6, 2024, Valisure, an independent testing laboratory, found high levels of benzene when BPO products were incubated at 37°C, 50°C, and 70°C.
anna.stasiia/AdobeStock

A newly published research letter in Environmental Health Perspectives further examined the testing methods used to detect benzene recently found in benzoyl peroxide (BPO)-containing acne products.1 On March 6, 2024, Valisure, an independent testing laboratory in Connecticut, found high levels of benzene when BPO products were incubated at 37°C (98.6°F: standard body temperature), 50°C (122°F: accepted pharmaceutical stability/shelf-life testing temperature), and 70°C (158°F: transportation or passenger vehicle temperature).2
As BPO is a commonly used over-the-counter (OTC) and prescription treatment for acne vulgaris, clinicians and consumers alike have expressed concerns about the effects of benzene contamination. Valisure has submitted a Citizen’s Petition to the US Food and Drug Administration (FDA) requesting an investigation and market withdrawal of BPO-containing products.3
In their research letter, Kucera et al reviewed the composition of BPO, the testing methods used by Valisure, and the results of the evaluated BPO products.
Background and Methods
BPO is a diacyl peroxide with bactericidal activity commonly used in OTC and prescription-strength acne treatments in up to 10% concentrations. According to Kucera et al, BPO is known to “thermally decompose to form 2 molecules of benzoyloxy radicals that can further decompose to benzoic acid or phenyl radicals with liberation of carbon dioxide.” Phenyl radicals can then produce end products of benzene, phenyl benzoate, and biphenyl.
Although the FDA conditionally “allows” 2 parts per million of benzene in drug products, there is truly no safe level of benzene. Technically, the FDA specifies that no drug products should contain benzene due to its toxicity, but that 2 parts per million is acceptable only for products that absolutely require benzene for manufacturing. Despite its carcinogenic concerns, high levels of benzene have been found in hand sanitizers, sunscreens, antifungal sprays, dry shampoos, and antiperspirants since 2021.
Valisure used orthogonal analytical techniques of gas chromatography-mass spectrometry (GC-MS) and selected-ion flow-tube MS (SIFT-MS) to evaluate benzene concentrations found within topical BPO products and gaseous benzene released into the air. Testing was performed at 37°C (98.6°F), 50°C (122°F), and 70°C (158°F).
Additionally, GC-MS analysis for native benzene was used to test 99 BPO-containing acne products. Valisure found that 94 BPO products had values well above 2 parts per million of benzene in an initial analysis.3
At the 2024 American Academy of Dermatology Annual Meeting in San Diego, California, Christopher Bunick, MD, PhD, presented brand new data highlighting benzene found in BPO products at day 0 without elevated temperatures. Of the 66 BPO products tested at day 0 at room temperature, 10 of the products had more than 10 parts per million of benzene, and 19 products had more than 2 parts per million of benzene.4
According to Bunick, associate professor of dermatology and physician-scientist at the Yale School of Medicine, the problem with BPO is degradation, not contamination.
Figure. Day 0 values of 5 BPO products incubated at 37 °C
Data provided by Valisure, LLC
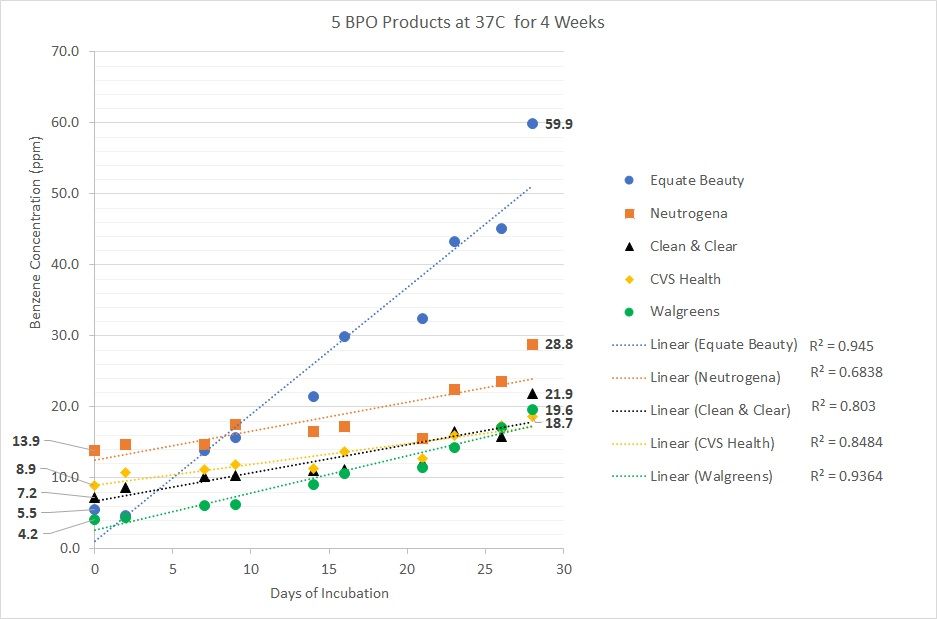
Bunick also highlighted Valisure'e data that at day 0 of incubating 5 BPO products at 37°C, human body temperature, for 4 weeks, Neutrogena's 10% BPO cleanser had 13.9 parts per million of benzene; CVS Health's 10% BPO face wash had 8.9 parts per million of benzene; Clean & Clear's 10% BPO cleanser had 7.2 parts per million of benzene; Equate Beauty's 2.5% BPO cleanser had 5.5 parts per million of benzene; and Walgreens' 10% BPO cream had 4.2 parts per million of benzene (Figure).
For its testing, Valisure selected single lots of 7 differently formulated and branded BPO products based on the availability of the products from national retailers. GC-MS testing included:
- Equate Beauty 2.5% BPO cleanser 4 oz (118 mL) UPC 681131200066
- Neutrogena 10% BPO cleanser 5 oz (148 mL) UPC 070501024638
- CVS Health 10% BPO face wash 6.6 oz (195 mL) UPC050428326930
- Walgreens 10% BPO cream 1.12 oz (33 mL) UPC049022657021
- Clean & Clear 10% BPO cleanser 5 oz (148 mL)UPC 38137-0032892
SIFT-MS testing included:
- Equate Beauty 10% BPO acne wash 5.5 oz (163 mL) UPC 681131436496
- Proactiv 2.5% BPO cleanser 4 oz (118 mL) UPC 842944102170
GC-MS Testing
“Benzene 13C6 (99.8% Sigma-Aldrich) was used for BPO studies. Dimethyl sulfoxide (DMSO; GC head-space grade, Thermo Fisher Scientific) was used for sample preparation and was separately tested with up to 10% BPO (98% Sigma-Aldrich) or benzoic acid (99.5% Sigma-Aldrich), and no benzene was observed following incubation up to 10 d at 37°C. The lower limit of detection (LLOD) was 10 ng (equivalent to 0.02 ppm in products), and the lower limit of quantitation (LLOQ) was 25 ng. The measurement uncertainty was determined to be 20%,” wrote Kucera et al.
Additionally, stability testing in a laboratory oven was performed on 5 BPO products at 37°C for 4 weeks and at 50°C for 3 weeks.
SIFT-MS Testing
Using a Syft Technologies Voice200ultra mass spectrometer, 2 common BPO products were incubated separately at 70°C for 16.7 hours using a “2-L DuoBath (Benchmark Scientific) that was placed in the chamber and filled with metallic Lab Armor Beads. The BPO products were removed from their external cardboard packaging, if present, and caps on the primary BPO product containers were not removed,” wrote Kucera et al.
C6H6 •NO+ and C6H+6 were used to measure benzene concentration before and during the incubation of BPO products in an environmental chamber with 3-s measurement cycles. Lastly, system sensitivity was evaluated by injecting 2 separate reference gasses, where benzene concentration was contained at 5 parts per million, into the chamber, “generating a 7-point curve with R2 values >0.995 and absolute concentrations within the expected variability of the reference standards. The LLOD and the LLOQ of benzene were calculated from the 2 separate reference gas calibration curves and resulted in Don Wolf & Associates LLOD of 1.55 ppb [parts per billion] and LLOQ of 4.70 ppb and GASCO LLOD of 1.64 ppb and LLOG of 4.97 ppb.”
Results
From its testing analysis, Valisure determined that all BPO products could form benzene, but that there is no evidence supporting acne products without BPO could form benzene. At day 28 of incubating BPO products at 37°C (standard body temperature), Equate Beauty’s 2.5% BPO cleanser had 59.9 parts per million of benzene; Neutrogena’s 10% BPO cleanser had 28.8 parts per million of benzene; Clean & Clear’s 10% BPO cleanser had 21.9 parts per million of benzene; Walgreen’s 10% BPO cream had 19.6 parts per million of benzene; and CVS Health’s 10% BPO face wash had 18.7 parts per million of benzene.
When using SIFT-MS during 70°C incubation for 16.7 hours on 2 BPO products to detect benzene in the air, Equate Beauty’s 2.5% BPO cleanser had 1160 parts per billion of benzene and Proactiv’s 2.5% BPO cream had 2724 parts per billion of benzene. According to Kucera et al, the SIFT-MS test “suggests that BPO products could emit substantial amounts of benzene from their original packaging prior to use.”
Kucera et al also noted that the US Environmental Protection Agency has found that a lifetime exposure to 0.4 parts per billion of benzene in the air can lead to one additional case of cancer in 100,000 exposed individuals.
“Solid BPO at purities up to 75% is known to be thermally stable to 98°C and various pharmaceutical additives are known to destabilize BPO. Therefore, reformulation with attention to benzene formation, could lead to BPO drug products that will not form benzene over time. Further investigation of BPO stability should be undertaken and regulatory actions should be considered in the interest of protecting public health,” concluded the authors.
References
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132(3):37702. doi:10.1289/EHP13984
- Andrus E, Bader K. Benzene found in various acne products; Valisure files petition with FDA to recall treatments. Dermatology Times. March 6, 2024. Accessed March 18, 2024. https://www.dermatologytimes.com/view/breaking-news-benzene-found-in-various-acne-products-valisure-files-petition-with-fda-to-recall-treatments
- Valisure citizen petition on benzene in benzoyl peroxide drug products. Valisure. March 5, 2024. Accessed March 18, 2024. https://assets-global.website-files.com/6215052733f8bb8fea016220/65e8560962ed23f744902a7b_Valisure%20Citizen%20Petition%20on%20Benzene%20in%20Benzoyl%20Peroxide%20Drug%20Products.pdf
- Bunick C. Discussion of new findings of benzene in BPO-containing products. Presented at: 2024 American Academy of Dermatology Annual Meeting; March 8-12, 2024; San Diego, CA.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














