- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Bristol Myers Squibb Presents 3-Year Safety and Efficacy Data of Deucravacitinib for PsO at EADV
Author(s):
Clinical response was maintained at 73.2% for PASI-75 in POETYK PSO long-term extension trial.
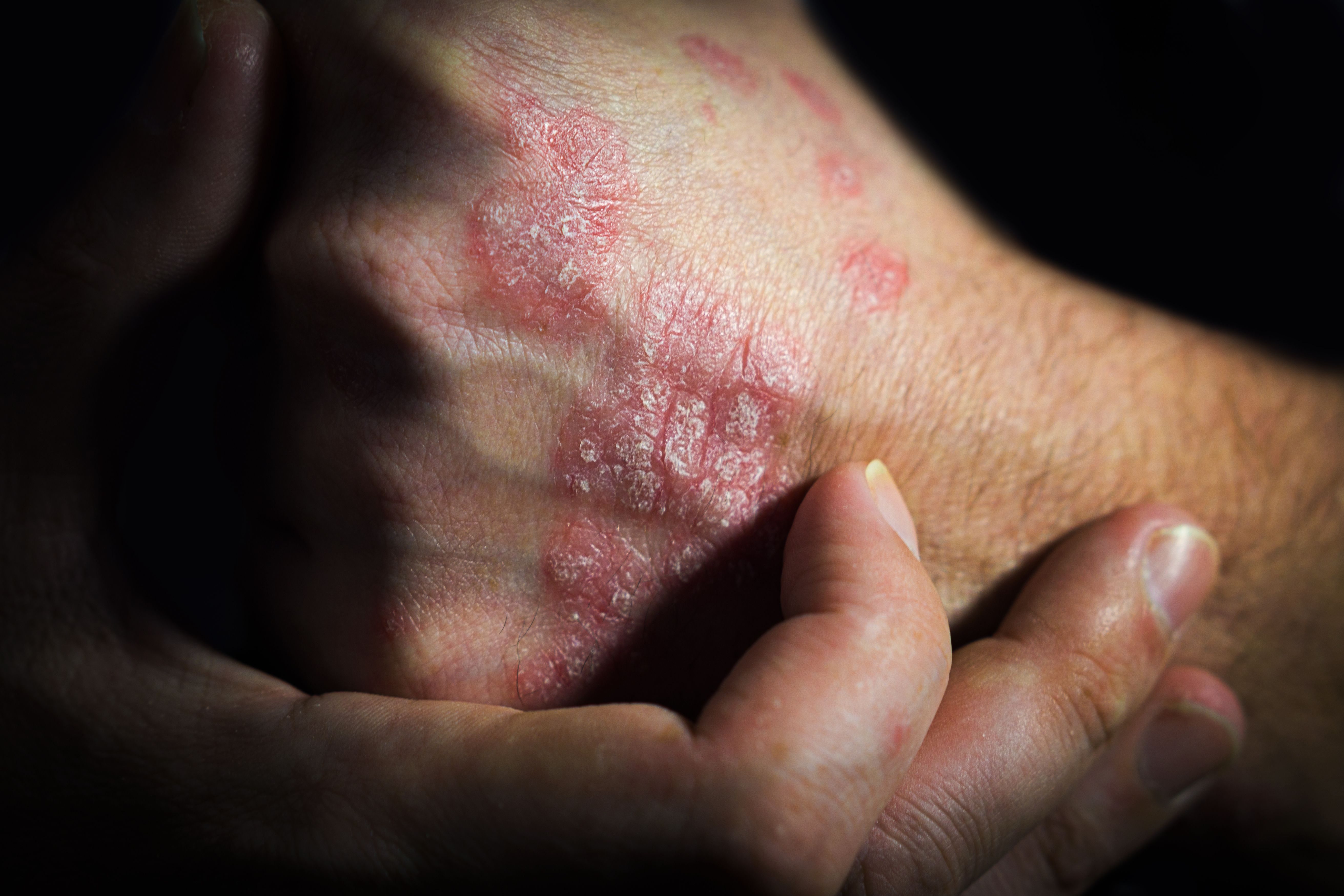
Bristol Myers Squibb recently presented positive 3-year results from the POETYK PSO long-term extension trial of deucravacitinib (Sotyktu) in adult patients with moderate-to-severe plaque psoriasisat the European Academy of Dermatology and Venereology (EADV) Congress in Berlin, Germany.1
Key findings at week 148 include:
- Psoriasis Area and Severity Index (PASI) 75: 73.2% of patients maintained a PASI 75 response
- PASI 90: 48.1% of patients achieved a PASI 90 response
- Static Physician’s Global Assessment (sPGA) 0/1: Approximately 54.1% of patients maintained an sPGA score of 0/1, indicating clear or almost clear skin
These results validate the long-term efficacy of once-daily deucravacitinib as the first and only TYK2 inhibitor available for adults with moderate-to-severe plaque psoriasis.
The safety analysis evaluated 1519 patients who received deucravacitinib across multiple trials. The analysis demonstrated a consistent safety profile with no increases in the rates of adverse events (AEs) or serious AEs over time. Additionally, no new safety signals emerged during the 3-year period.
“As the leader in TYK2 innovation, Bristol Myers Squibb continues to advance our long-term understanding of our first-in-class, oral Sotyktu treatment for plaque psoriasis and explore its full potential across serious immune-mediated diseases,” said Roland Chen, MD, senior vice president and head of immunology, cardiovascular, and neuroscience development at Bristol Myers Squibb, in the news release. “These new data validate the potential of Sotyktu to provide long-term, clinically relevant improvement for individuals living with moderate-to-severe plaque psoriasis.”
The efficacy analysis included 513 patients who received continuous deucravacitinib treatment from the pivotal trials through the long-term extension trial. These patients showed sustained response rates for PASI 75, PASI 90, and sPGA 0/1, confirming the durability of the treatment over a 3-year period.
The safety analysis revealed that cumulative exposure-adjusted incidence rates (EAIRs)/100 PYs for AEs, serious AEs, discontinuation due to AEs, herpes zoster, malignancies, major adverse cardiovascular events, venous thromboembolism, and deaths either remained similar or decreased over time compared to 2-year rates. These findings demonstrate the stable safety profile of deucravacitinib in the long term.
“These new, positive, three-year results reinforce the long-term efficacy and well-established safety profile of once-daily Sotyktu, the first and only TYK2 inhibitor available, and add to our confidence in its role as an oral treatment of choice for adults with moderate-to-severe plaque psoriasis,” said April Armstrong, MD, MPH, clinical investigator in the POETYK PSO clinical trial program and professor and chief of dermatology at the University of California, Los Angeles, in the news release. “For my patients, more days of relief from this chronic disease mean that they can focus on other aspects of their lives, and these POETYK PSO long-term data add to the evidence that we have the ability to offer a new standard of care to patients seeking an oral treatment option.”
These new 3-year results reinforce the position of deucravacitinib as a promising oral treatment for moderate-to-severe plaque psoriasis. Recently, the September issue of Dermatology Times included a year in review of deucravacitinib and its efficacy in psoriasis management. Neal Bhatia, MD; Melinda Gooderham, MD; and Lauren Miller, PA-C, provided expert insights on patients' experiences with deucravacitinib and what dermatology clinicians are looking forward to long-term.
Reference
- Sotyktu (deucravacitinib long-term data demonstrate durable efficacy and consistent safety for up to three years in moderate to severe plaque psoriasis. Bristol Myers Squibb. News release. October 11, 2023. Accessed October 11, 2023. https://news.bms.com/news/details/2023/Sotyktu-deucravacitinib-Long-Term-Data-Demonstrate-Durable-Efficacy-and-Consistent-Safety-for-up-to-Three-Years-in-Moderate-to-Severe-Plaque-Psoriasis/default.aspx
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.