
- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Chronic Immune-Related Adverse Events Common in Anti-PD-1 Therapy for Melanoma
Author(s):
Endocrine toxic effects were more likely than nonendocrine to become chronic and continue at last follow-up.
To assess the incidence, characteristics, and long-term outcomes of chronic immune-related adverse events (irAEs) caused by adjuvant anti-programmable cell death-1 (anti-PD-1) therapy, researchers conducted a retrospective, multicenter cohort study and found that irAEs were common and affected multiple organ systems.1
Maris/AdobeStock
Goodman et al collected data from 6 academic medical centers in the United States and Australia. Patients who received at least 1 dose of adjuvant anti-PD-1 therapy with at least 18 months of follow-up were included. Acute irAEs were those that arose during treatment, delayed irAES arose within 6 months of treatment cessation, and chronic irAEs were those that continued at least 3 months after treatment cessation.
Median age of the 318 patients in the study was 64 years and 59.7% were male. Patients were treated with adjuvant anti-PD-1 therapy for advanced and metastatic melanoma. Nivolumab was given to 255 (80.2%) patients andpembrolizumab was given to 63 (19.8%) patients. The median duration of treatment was 315 days.
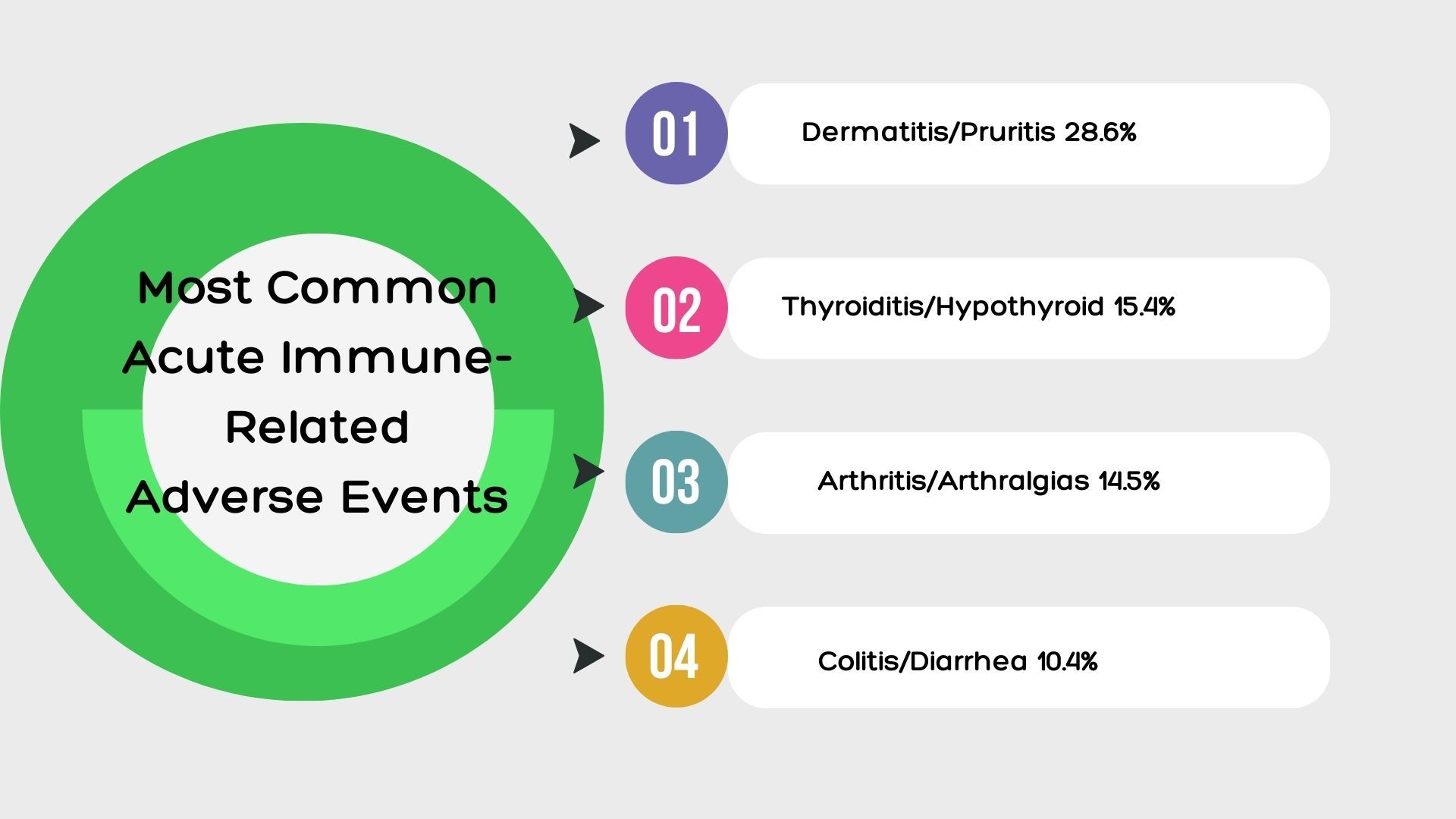
Of the 318 patients, 226 developed acute irAEs and 53 developed delayed toxic events. Most acute irAES were grade 2 or higher (144 patients) and 114 required steroids. Dermatitis or pruritis (28.6%), thyroiditis or hypothyroid (15.4%), arthritis or arthralgias (14.5%), and colitis or diarrhea (10.4%) were the most common acute irAEs.
Acute irAEs, including delayed irAEs, that were likely to become chronic were adrenal insufficiency, hypophysitis, thyroiditis/hypothyroid, neuropathy, and nephritis.
Of the 318 patients, 147 (46.2%) developed chronic toxic effects, with 74 of those patients experiencing at least a grade 2, and 6 of those patients experiencing a grade 3 to 5. Of the patients who developed chronic toxic effects, 56 (38.1%) required corticosteroids. One hundred patients (68%) experienced symptomatic chronic toxic effects.
Of the 75 patients with acute endocrine irAEs, 64 (85.3%) became chronic, and 11 of those also had nonendocrine irAEs.
Resolution of chronic irAEs at last follow-up occurred for 54 of 147 patients (36.7%) with extended follow-up (median, 1057 days). Of the 147 with chronic toxic effects, 93 (63.3% of the cohort) had ongoing effects. Chronic irAEs resolved in 14.4 to 31.5 months from the initiation of anti-PD-1 and 8.1 to 20.7 months from the cessation of anti-PD-1 therapy.
Of 93 patients with persistent toxic effects at last follow-up, 55 (17.3% of original cohort) were grade 2 or higher and 41 (12.9% of original cohort) continued to be symptomatic. The majority of persistent irAEs were hypothyroid (40.9%), adrenal insufficiency (8.6%), arthritis (19.4%), dermatitis or pruritis (.7%), and hypophysitis (8.6%).
Of the 6 patients with chronic grade 3 to 5 irAEs, 3 showed improvement by last follow-up, 2 continued to have ongoing irAEs at last follow-up, and 1 had resolved.
Endocrine toxic effects were more likely to become chronic and continue at last follow-up than nonendocrine toxic effects, but other more symptomatic irAEs persisted at low rates individually, including cutaneous, rheumatologic, oral, and ocular events.
Goodman et al concluded that “Insights into the long-term impact of adjuvant anti–PD-1 therapy are crucial to optimize patient outcomes as these agents are used across different tumor types. The high prevalence of chronic irAEs suggests the importance of considering the risk-benefit ratio when initiating adjuvant therapy and the need for prolonged monitoring and proactive management of irAEs.”
Reference
- Goodman RS, Lawless A, Woodford R, et al. Extended follow-up of chronic immune-related adverse events following adjuvant anti–PD-1 therapy for high-risk resected melanoma. JAMA Netw Open. 2023;6(8):e2327145. doi:10.1001/jamanetworkopen.2023.27145
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.










2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.




