- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Clinical Response Correlates With Serum Infliximab in Hidradenitis Suppurativa
Author(s):
A recent study supports a degree of correlation between trough serum concentration of infliximab and clinical response to infliximab in patients with the condition.
In patients with hidradenitis suppurativa (HS), serum infliximab levels and clinical response may go hand-in-hand.
Lea/AdobeStock
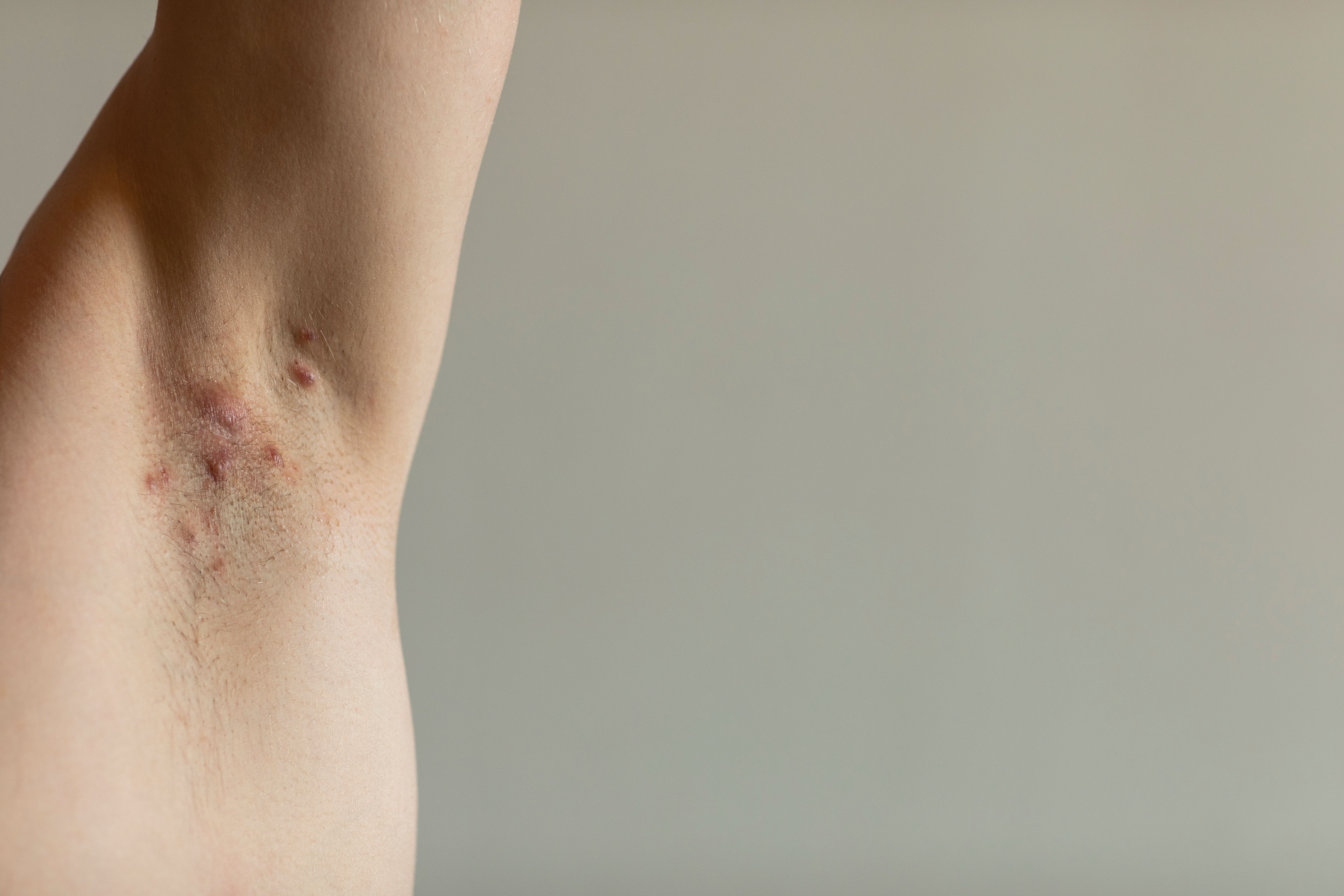
According to a recent study,1 researchers determined a degree of correlation between the 2 factors, citing a lack of research supporting or evaluating the relationship between clinical response and infliximab pharmacokinetics.
From January 2013 to January 2022, researchers conducted a retrospective study at a dermatology tertiary care center in Paris, France. Patients included in the study (n=45) were adult patients with moderate-to-severe HS who had received at least 1 dose of infliximab serum during the aforementioned time frame. Moderate-to-severe disease was defined as patients meeting criteria for Hurley stage II or III.
Researchers collected patient data from the care center’s pharmacy database and retrospectively analyzed data, including sex, age, body mass index, smoking status, comorbidities, family history of HS, and characteristics of HS. Some characteristics were determined using blood sampling. These included:
- Localizations
- Hurley stage
- Hidradenitis Suppurativa Clinical Response (HiSCR) score
- Hidradenitis Suppurativa Physician’s Global Assessment (HsPGA)
- Prior treatment history and treatment optimization
- Biological data (C-reactive protein serum levels, serum leucocytes count, infliximab serum levels, antidrug antibodies)
- Infliximab treatment modalities (dosage and frequency)
Initially, all participants were treated with 5 mg/kg of infliximab at weeks 0, 2, and 6. Then, 54% of participants began a treatment regimen of 5 mg/kg of infliximab administered every 8 weeks. Before each infliximab infusion, researchers performed pharmacokinetic and antidrug antibody assessments.
Of the entire study population (n=45), 22 enrolled patients had received an infliximab serum dosage and HiSCR evaluation between weeks 12 and 24. Of these, 18 participants were considered HiSCR non-responders, and 4 were considered HiSCR responders. Of the entire population, 2 participants were excluded due to missing HiSCR, and 21 were excluded due to a lack of infliximab serum dosage between at weeks 12 and 24.
At baseline, non-responder and responder group patients did not have statistically significant differences in characteristics. However, though not statistically significant in number, non-responding participants tended to demonstrate signs of more severe disease, a more inflammatory phenotype, and more associated inflammatory comorbidities.
Between weeks 12 and 24, the median trough serum concentration was higher in the responder group (n=14.8µg/mL) than in the non-responder group (n=1.6 µg/mL). Antidrug antibodies were only detected in participants (n=3) in the non-responder group.
According to HiSCR scores, trough serum infliximab less than 7 µg/mL at weeks 12 through 24 were significantly associated with an absence of clinical response.
Potential study limitations, as noted by researchers, include the study’s retrospective and monocentric nature as well as its small sample size. Furthermore, trough serum infliximab may have been assessed more frequently in HiSCR non-responding participants.
“The analysis in this study suggests that the threshold infliximab level that predicts response may be closer to ankylosing spondylitis and Crohn’s disease than psoriasis,” study authors wrote. “A prospective study with infliximab pharmacokinetic monitoring would be necessary to determine an infliximab threshold of efficacy that could be helpful in the management of patients with HS.”
Reference
- Benassaia E, Bouaziz J, Jachiet M, et al. Serum infliximab levels and clinical response in hidradenitis suppurativa. JEADV Clin Pract. Published online 2023. doi:10.1002/jvc2.139
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









