- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
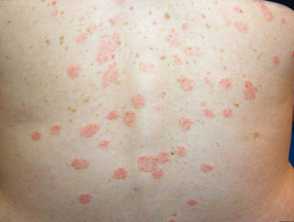
COVID-19 Vaccination May Increase Risk of Pityriasis Rosea, Similar Eruptions
Author(s):
Additional studies are needed to adopt preventative measures and identify patients who may be deemed "at risk."
As COVID-19 vaccination rates increased, dermatologists began encountering various cutaneous reactions, including viral reactivations.
A study recently published by Dovepress focuses on pityriasis rosea (PR) and PR-like eruptions following COVID-19 vaccination, assessing their prevalence, possible pathogenetic mechanisms, and causal correlations.
A comprehensive literature review using multiple databases identified 113 cases of PR following COVID-19 vaccination.
In conducting this review, an extensive literature search was carried out using various databases, including PubMed, Embase, Google Scholar, Cochrane Skin, EBSCO, and MEDLINE, up until October 27, 2023. The search utilized specific keywords related to COVID-19, vaccination, adverse events, vaccine, skin manifestations, cutaneous side effects, mRNA, viral-vector, and various vaccine names.
The review included meta-analyses, reviews, editor letters, real-life studies, case reports, and case series, with a focus on selecting the most relevant documents. Articles discussing PR and PR-like eruptions following vaccines not approved by the European Medicines Agency were excluded.
The research involved a thorough examination of the texts and abstracts of collected articles, and additional efforts were made to include relevant articles by evaluating the references of the selected manuscript. Only English language manuscripts were considered, and the article is based on previously conducted studies, with no involvement of human participants or animals by the authors.
BNT162b2 was the most common vaccine associated with PR, and the majority of cases occurred after the first dose. The median time from vaccination to PR onset was 9 ± 6.3 days.
No severe cases or PR occurrences were reported after the third vaccine dose.
Potential study limitations included the inadequate number of patients for robust correlation assessment and the absence of clinical trials.
The study suggests that exposure to viral antigens during vaccination may boost the cell-mediated immune response, potentially leading to inflammation and the reactivation of latent viral infections, including HHV6 and HHV7. While PR and PR-like eruptions following COVID-19 vaccination are rare, study authors wrote that clinicians should be vigilant in recognizing and reassuring patients about these cutaneous reactions.
"Globally, the number of cases of PR and PR-like eruption is extremely low if compared with the number of vaccines administered, leading to the impossibility to demonstrate that COVID-19 vaccination may increase the risk of PR and PR-like eruption development," they wrote. "In our opinion, clinicians should keep in mind the possibility of the development of this cutaneous disease following vaccination. Certainly, more studies are needed to identify 'at-risk' patients and adopt preventative measures. Surely, vaccination should not be discouraged."
Reference
Potestio L, Martora F, Cacciapuoti S, et al. Pityriasis rosea and pityriasis rosea-like eruption following COVID-19 vaccination: A narrative review. Dovepress. Published online January 8, 2024. Accessed January 12, 2024. https://doi.org/10.2147/CCID.S447834
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.