- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Cynosure Announces Clearance for RF Microneedling Device
Author(s):
Cynosure’s four-mode radiofrequency microneedling device, Potenza, offers the ability to perform both deep and shallow treatments with a single system.
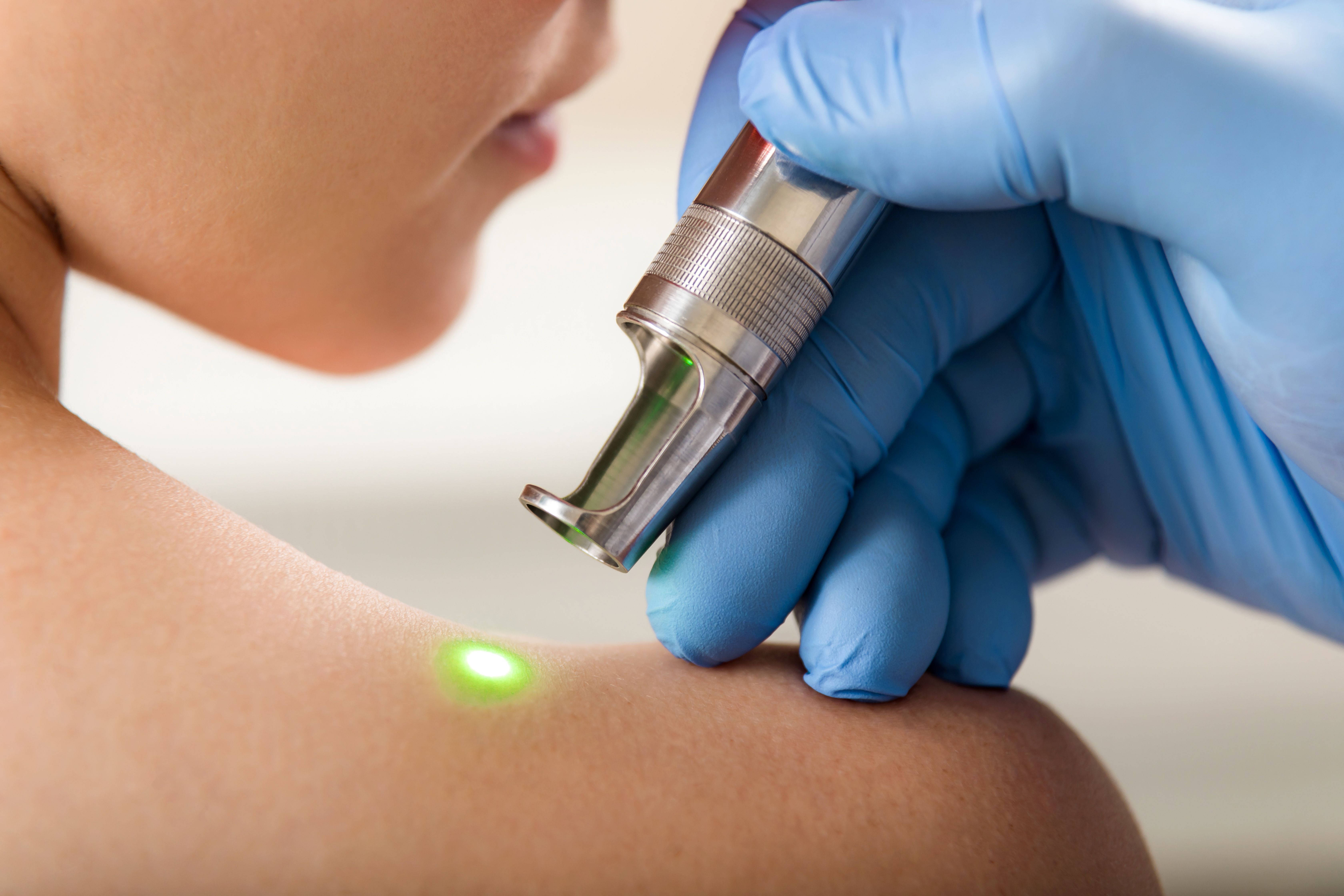
Cynosure, a leader in energy-based aesthetic and medical treatment devices, recently announced the U.S. Food and Drug Administration (FDA) clearance of Potenza, a four-mode radiofrequency (RF) microneedling system.
The first FDA cleared device of its kind, Cynosure says Potenza offers a more customized microneedling experience with four mode options: monopolar or bipolar, delivered at either 1 MHz or 2 MHz frequency, according to the press release.
“Potenza treatments use ultrafine needles and radiofrequency energy to penetrate the top layer of the skin and trigger the body's natural healing process to regenerate new collagen and elastin,” according to the company. “Unlike some other skin revitalization options on the market, Potenza treatments can be performed on all skin types, anywhere on the body and any time of year.”
This offers the ability to perform both deep and shallow treatments with a single system.
Using the device’s monopolar RF mode, practitioners can deliver energy treatments across large areas of tissue for deep heating and skin tightening, while the bipolar RF mode offers concentrated energy for the treatment of superficial tissue.
According to the company, Potenza treatments can be performed on all Fitzpatrick skin types, anywhere on the body, at any time of the year.
The system comes equipped with Tiger Tip technology which, the company describes as the “first semi-insulated needles of its design which allow practitioners to expand the treatment zone and address more tissue per treatment, which translates to quicker sessions for patients, without sacrificing the epidermis,” according to the release.
A single-needle handpiece is also included.
Potential side effects may include temporary redness, tingling and a burning sensation while receiving treatment.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.