- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
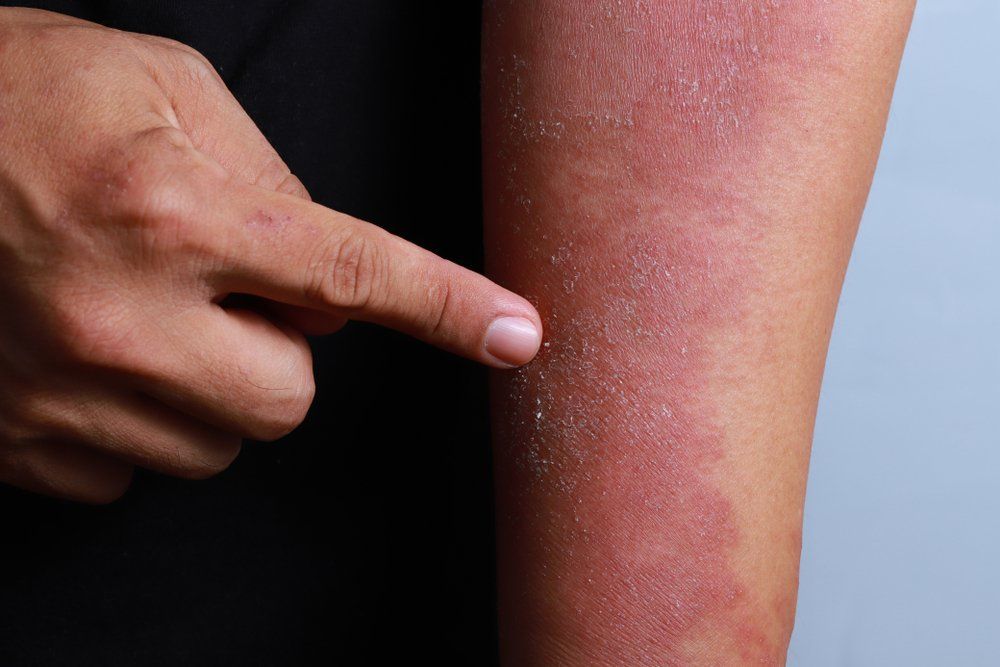
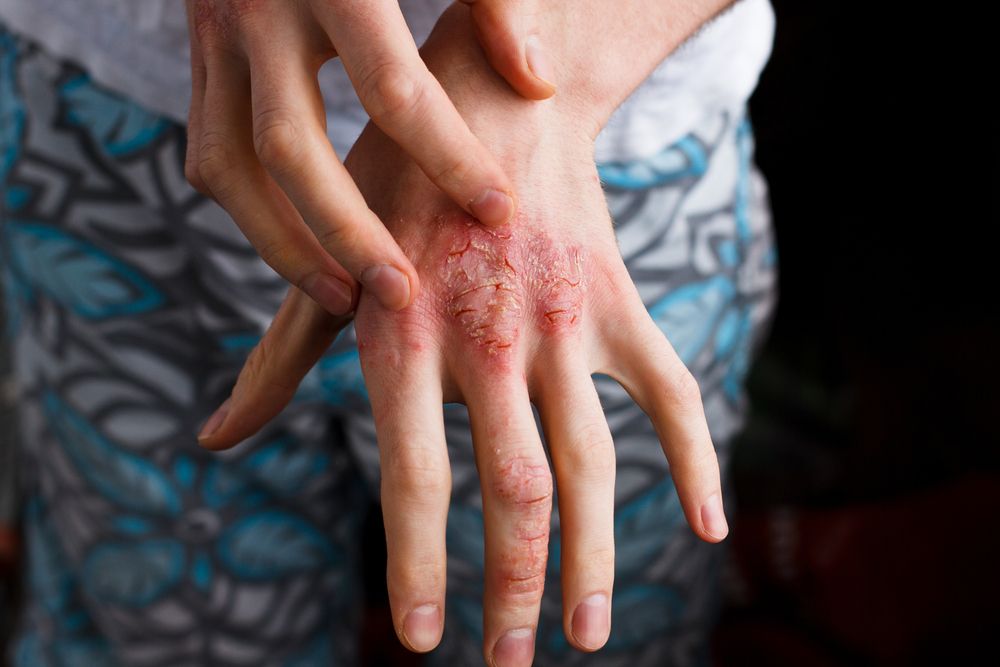
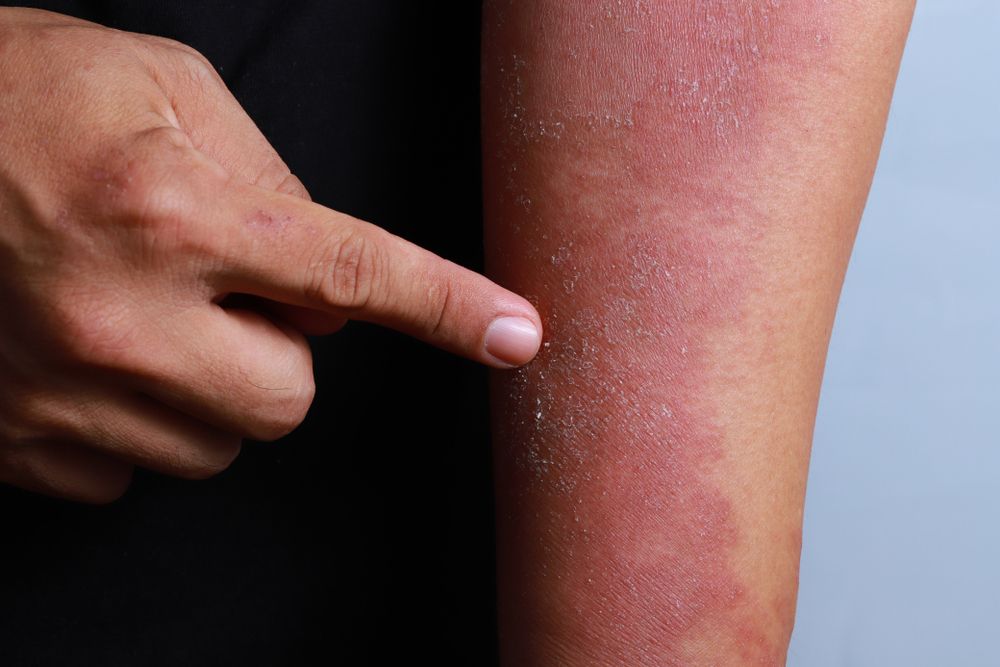
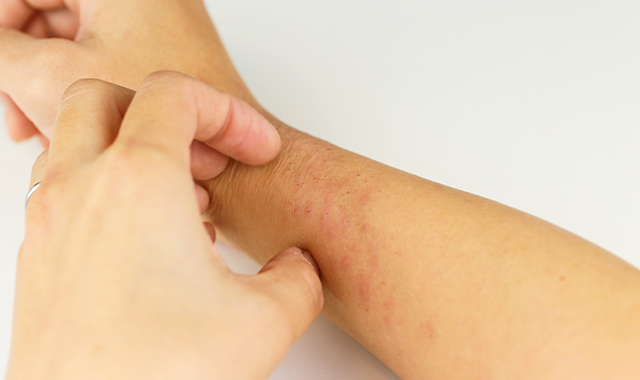
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Dupilumab demonstrates promising results
Author(s):
The systemic therapy dupilumab met primary endpoints in two phase 3 studies on adults with in inadequately controlled moderate-to-severe atopic dermatitis.
The systemic therapy dupilumab met primary endpoints in two phase 3 studies on adults with inadequately controlled moderate-to-severe atopic dermatitis, according a recent release by Regeneron Pharmaceuticals and Sanofi.
In the identically designed SOLO 1 and SOLO 2 trials, monotherapy with investigational dupilumab significantly improved disease severity, skin clearing, itching, quality of life and mental health.
"These data provide strong evidence that the IL-4 and IL-13 signaling pathway is a fundamental driver of inflammation in atopic dermatitis. Dupilumab is the first in a new class of immunotherapies – in these 16 week trials, dupilumab blocked the aberrant activation of this pathway, resulting in significant efficacy without evidence of immune-suppressing side effects," George D. Yancopoulos, M.D., Ph.D., chief scientific officer of Regeneron and president of Regeneron Laboratories, says in the release.
Researchers studied 1,379 subjects with moderate-to-severe disease who were inadequately controlled with topical medications or were not good candidates for topical treatment. They assessed subjects using the Investigator's Global Assessment (IGA) scale, ranging from 0 (clear) to 4 (severe). Study participants had IGA scores of 3 or 4 at baseline. They also assessed subjects with the Eczema Area and Severity Index (EASI).
Subjects were randomized to receive dupilumab 300 mg subcutaneously once per week; dupilumab 300 mg subcutaneously every two weeks; or placebo for 16 weeks following an initial dupilumab loading dose of 600 mg subcutaneously, or placebo.
In SOLO 1, 37% of patients who received 300 mg of dupilumab weekly and 38% who received the 300 mg of the investigational drug every two weeks achieved IGA 0 or 1, compared with 10% in the placebo group. In SOLO 2, 36% of those receiving the weekly and every-two-week regimens achieved IGA 0 or 1 compared to 8.5% of controls.
EASI measures improved 72% from baseline in both the once-weekly and every-other-week treatment groups in SOLO 1, compared to 38% for placebo. In SOLO 2 EASI improved 69% in the weekly dose group and 67% among those who received dupilumab every two weeks, compared to 31% in placebo.
A secondary endpoint for the United States studies was the percentage of patients who achieved EASI-75. In SOLO 1, 52.5% in the weekly dupilumab treatment group and 51% in the every two weeks treatment group achieved EASI-75, compared to 15% of those in the placebo group. In SOLO 2, researchers reported 48% of patients in the weekly group and 44% in the every two weeks treatment group achieved EASI-75, compared to 12% in the placebo group.
NEXT: Adverse event rates
Adverse events
Overall adverse event rates, in the short-term studies, were comparable between the dupilumab groups and placebo. The rate of serious adverse events was one to three percent for dupilumab and five to six percent for placebo.
The FDA granted dupilumab 'Breakthrough Therapy' designation for atopic dermatitis. Sanofi’s President of global research and development Elias Zerhouni, M.D., says in the release that the company plans to submit a regulatory application in the third quarter of 2016.
Houston, Texas, dermatologist Rajani Katta, M.D., tells Dermatology Times that she is excited to see study results that support another potential option for the treatment of patients with moderate to severe atopic dermatitis.
“The severe itching and widespread skin involvement that are often present in this condition really impact multiple measures of quality of life, and we've definitely needed additional treatment options,” Dr. Katta says. “Currently, if patients are not responding to topical steroids or topical immunomodulators, then our options are limited. Phototherapy is time-consuming, and may not be readily available. Systemic immunosuppressive medications act to broadly suppress the immune system. This means that patients on one of these medications require careful observation for potentially severe side effects, as well as ongoing laboratory monitoring.”
Dupilumab, Dr. Katta says, represents a breakthrough because it is a systemic medication that targets specific immune system pathways that drive atopic dermatitis.
“The initial studies look very promising in terms of efficacy and safety profile, as compared to placebo. Of course, as with all medications that target immune system pathways, I'm concerned about long-term safety data, and that's something I'll be watching carefully,” she says.
Disclosure: Dr. Katta reports no relevant disclosures.