- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
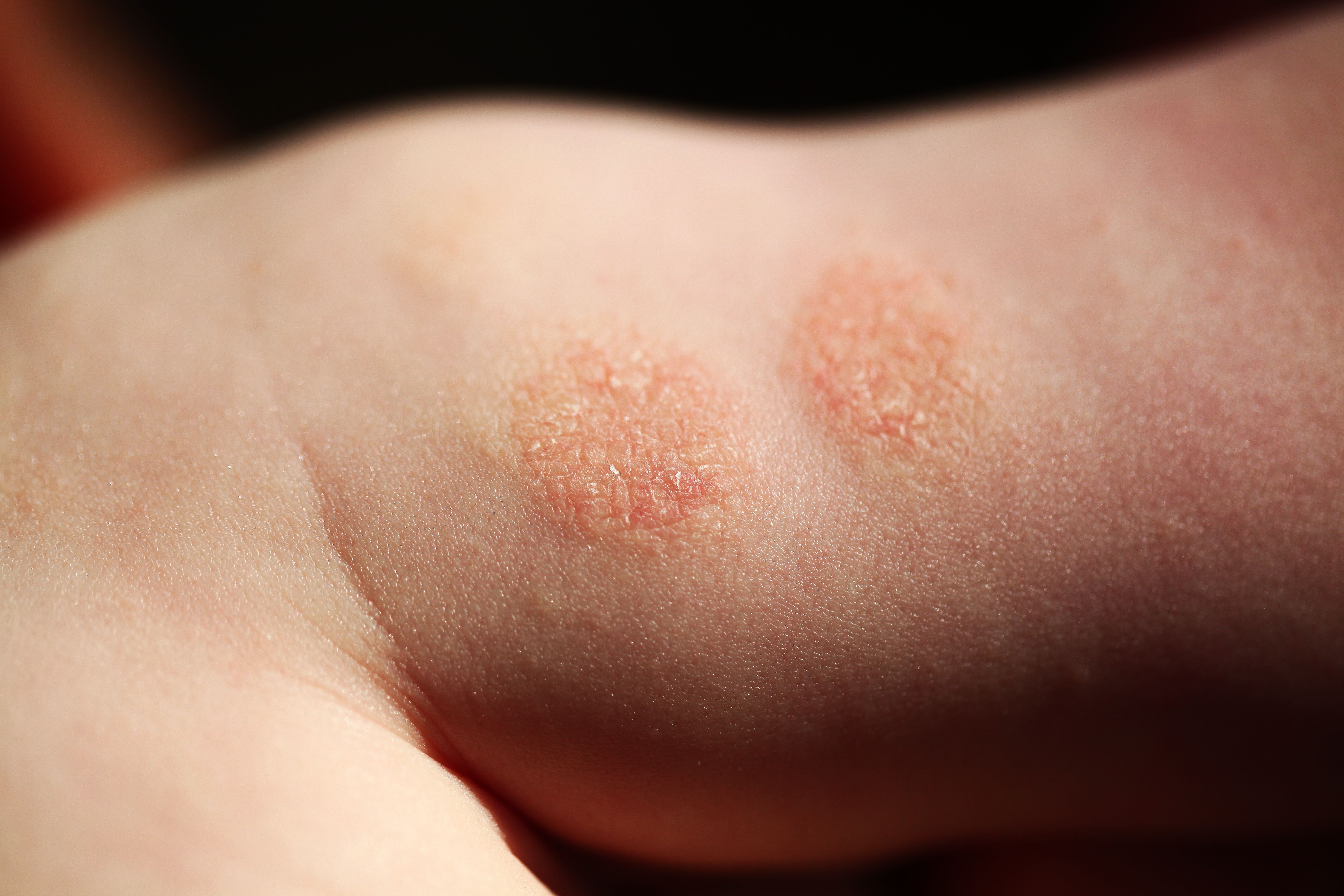
Dupilumab Effective in Reducing New Allergies and Slowing Atopic March
Author(s):
Findings indicate no rebound effect when participants discontinue dupilumab.
To determine whether treatment with dupilumab could reduce the acquisition of new allergies or the worsening of existing allergies, researchers applied a meta-analytical methodology to a large atopic dermatitis (AD) clinical trial database. Their findings showed that patients treated with dupilumab had a 34% reduction in new or worsened allergies (Incidence rate ratio[IRR] 0.66; 95% CI, 0.52-0.84) and a 37% reduction in new allergies (IRR 0.63; 95% CI, 0.48-0.83),1 a positive step in slowing the atopic march.
Children who produce IgE antibodies in response to environmental stimuli have a higher incidence of atopic march than those who do not. AD is strongly associated with food allergies and inhalant allergens.
Controlling the signaling of the cytokine IL-4 may be an important treatment option for halting the atopic march. Dupilumab is a monoclonal antibody that blocks IL-4R and has been effective in treating moderate to severe AD and asthma, as well as other diseases caused by type 2 inflammation.
Researchers gathered data from 12 randomized, placebo-controlled, double-blind, parallel-grouped trials looking at the use of dupilumab in the treatment of AD. The analysis reviewed studies with a duration of 4-52 weeks (1359 patient-years; n = 2296 dupilumab, n = 1229 placebo) and a total of 3525 patients. The median age at which participants began to show signs of AD was 2 years old. The median age of study participants was 35.
All study participants had moderate to severe AD at baseline that was not controlled with topical medication, an Investigator’s Global Assessment severity score of at least 3 (5-point scale; graded 0-4), and an Eczema Area and Severity Index (EASI) score of 16 or higher (maximum of 72).
Dosing across the 12 studies ranged from 100 mg of dupilumab every 4 weeks to 300 mg each week, with most patients receiving 300 mg either each week or every 2 weeks.
Researchers assessed allergic treatment-emergent adverse events (TEAEs) to determine if new allergic conditions arose and to assess the effectiveness of dupilumab on existing allergic diatheses. As all participants had AD at baseline, terms that mapped to AD were excluded from the assessment of atopic march.
Allergic burden at baseline was comparable between treatment groups, P = .672, and the average baseline concomitant allergic burden was 3.4 categories (excluding AD).
Of the 17 categories researchers evaluated as contributing to allergic TEAE events, asthma, pruritus, and urticaria showed the most positive responses to treatment with dupilumab.
The analysis showed that atopic march continues into adulthood and that dupilumab can interfere with the progression of atopic march. The participants taking dupilumab exhibited fewer new allergies and a decrease in the worsening of existing allergies than those on a placebo. Findings also suggest that the benefit of treatment with dupilumab is greater for younger patients (<18), those with an early onset of AD (<2), those with more severe AD at baseline, and those with asthma at baseline.
Researchers also found no rebound effect after discontinuing treatment with dupilumab, although longer studies will need to be done to confirm this finding. Those treated with dupilumab also showed significant improvement in disease severity, particularly in serum IgE levels.
This meta-analysis showed that dupilumab may be effective in slowing the atopic march.
Reference
- Geba GP, Li D, Xu M, et al. Attenuating the atopic march: Meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J Allergy Clin Immunol. 2023;151(3):756-766. doi:10.1016/j.jaci.2022.08.026 https://www.jacionline.org/article/S0091-6749(22)01176-9/fulltext
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.