- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
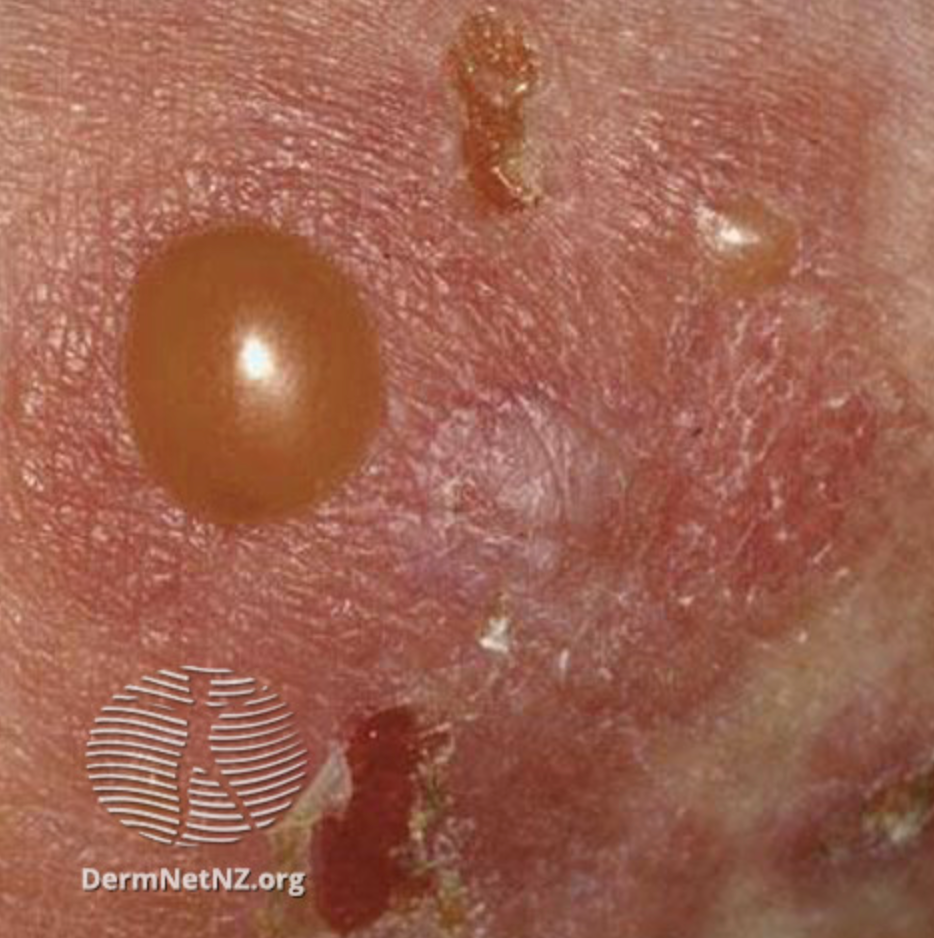
Dupilumab Successfully Treated Bullous Pemphigoid in Retrospective Study
Author(s):
Few adverse events resulted from treatment with dupilumab in 64 weeks of observation.
In a retrospective cohort study of patients with bullous pemphigoid (BP), researchers found that dupilumab resulted in a reduction of symptoms within 4 weeks.1 Studies from January 1, 2021, to July 31, 2022, of 146 patients aged 64 to 85 years were included.
The primary outcome measure was the proportion of participants achieving control of the disease, defined as absence of new lesions and pruritis, along with a healing of existing lesions, within 4 weeks. Secondary outcomes included complete remission rate, relapse within 64 weeks, adverse events (AEs), and changes in measurements of Bullous Pemphigoid Disease Area Index (BPDAI) scores, itching numerical rating scale (NRS) scores, and laboratory results.
The multicenter study of adults with BP included 6 dermatology departments across China. The patients were treated with an initial 600 mg dose of dupilumab, followed by 300 mg of dupilumab every 2 weeks. To be included in the study, at least 4 weeks of follow-up was required following the initial dose.
At initial dose, based on BPDAI scores, 6.2% of patients were considered to have mild BP, 24% as moderate, and 69.9% as severe. Concomitant medications included systemic steroids (58.9%), minocycline (57.5%), and topical steroids (21.2%).
After 4 weeks of treatment with dupilumab, 87% achieved disease control and 74.7% of those occurred within 2 weeks. Of the patients achieving control within 4 weeks, 60.6% received oral steroids concurrently. Complete remission was achieved by 35.6% of patients during the observation period.
Among the 94 participants who continued follow-up for more than 16 weeks, 43.6% changed to taking 300 mg of dupilumab every 4 weeks, 9.6% continued with treatment every 2 weeks, and 46.8% stopped treatment with dupilumab at or before 16 weeks. Of these, 1 patient (2.4%) relapsed after reducing the frequency but none of the patients who continued the original regimen relapsed. After discontinuing dupilumab, 90.9% of patients continued with stable disease status and 9.1% relapsed. “The cumulative relapse rate at weeks 16, 32, 48, and 64 were 6.6%, 9.5%, 17.1%, and 30.9%, respectively.”1
Of the 53.4% of patients who stopped taking dupilumab during the observation period, 64.1% stopped due to well-controlled BP, while 20.5% discontinued because of poor effectiveness, 7.7% discontinued because of AEs, and another 7.7% stopped due to economic concerns.
Among the 26.7% of patients who reported AEs, most were mild, and patients continued with therapy. The most common AEs were skin and soft tissue infections, followed by pneumonia.
Researchers concluded that dupilumab was an effective treatment for BP with minimal AEs.
Reference
- Zhao L, Wang Q, Liang G, et al. Evaluation of dupilumab in patients with bullous pemphigoid. JAMA Dermatol. Published online August 02, 2023. doi:10.1001/jamadermatol.2023.2428 https://jamanetwork.com/journals/jamadermatology/fullarticle/2808114?guestAccessKey=c146147a-bebf-4650-8abf-d4b032786b54&utm_source=silverchair&utm_medium=email&utm_campaign=article_alert-jamadermatology&utm_content=olf&utm_term=080223
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.