- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Dupilumab tames AD in difficult-to-treat patients
Author(s):
Dupilumab given concomitantly with topical corticosteroids significantly improved signs and symptoms of atopic dermatitis (AD) in adults refractory to or intolerant of cyclosporine A, a study shows.
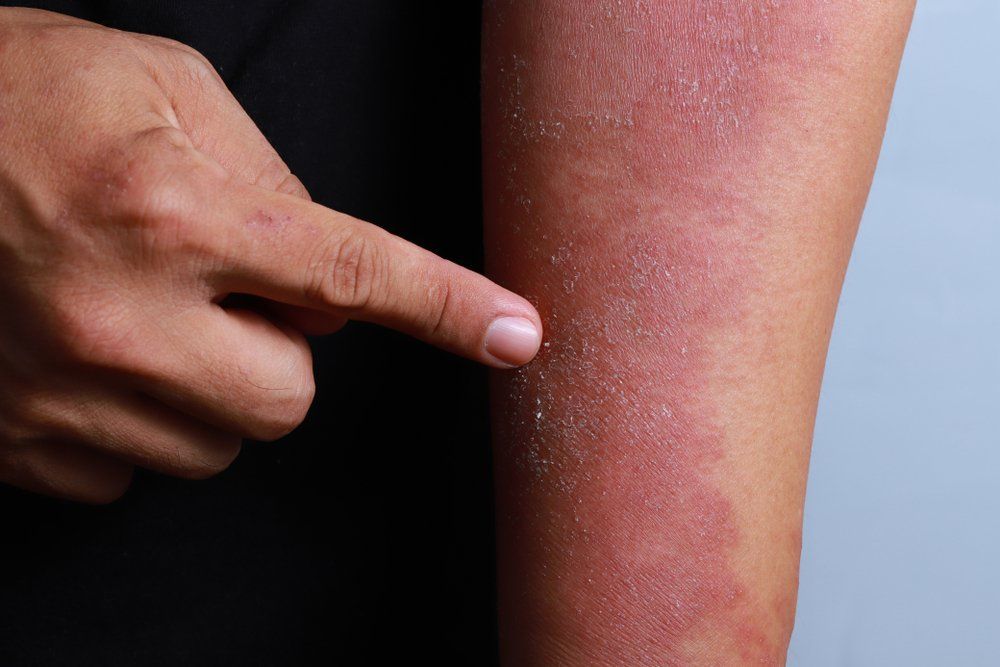
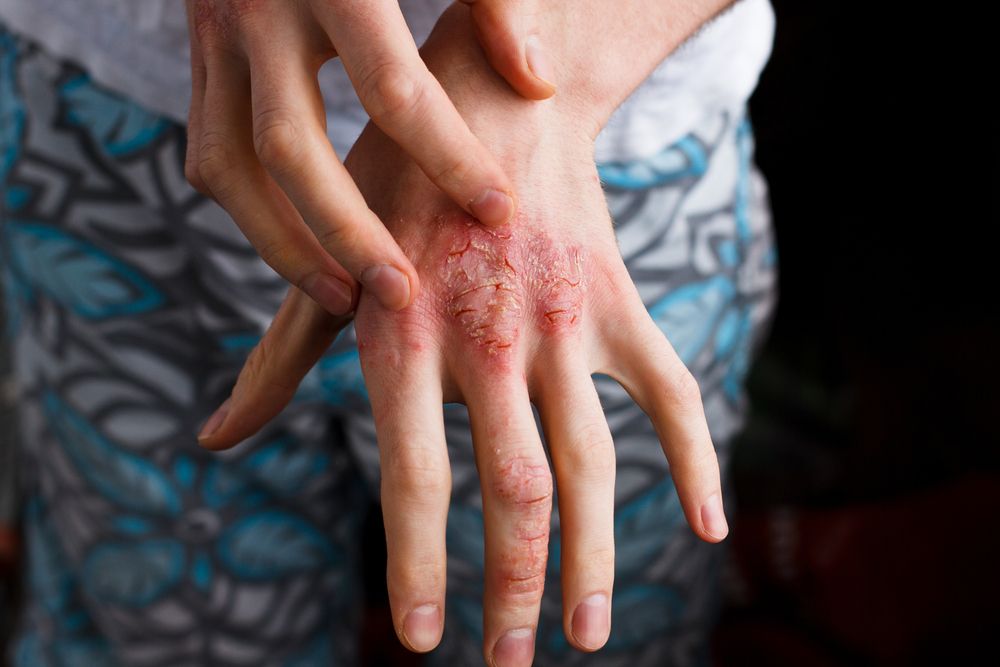
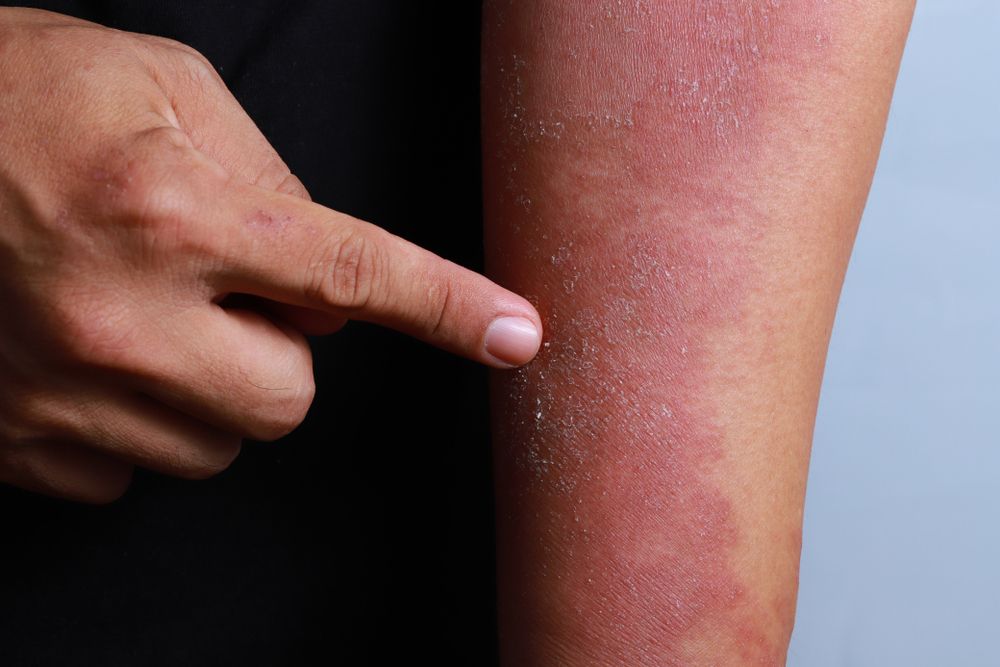
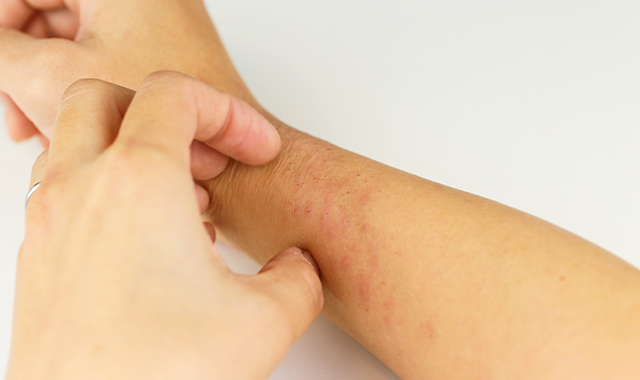
GENEVA-In a difficult-to-treat patient population, 16 weeks of dupilumab given concomitantly with topical corticosteroids significantly improved signs and symptoms of atopic dermatitis (AD) in adults refractory to or intolerant of cyclosporine A, according to results of a phase 3 clinical trial known as CAFÉ.
Reductions in lesion scores, pruritus, and patient-reported outcomes were observed as early as two weeks after starting dupilumab patients in whom topical corticosteroids cyclosporine A was ineffective at controlling their symptoms or who could not tolerate corticosteroids, reported Marjolein de Bruin-Weller, M.D., Ph.D., at the European Academy of Dermatology and Venereology Congress held this summer.
“These data support the use of dupilumab in adult patients with moderate to severe AD who have previously used cyclosporine A and stopped it due to intolerance or lack of efficacy or who are not candidates for cyclosporine A because of medical conditions or use of contraindicated concomitant medications,” said Dr. Bruin-Weller, a dermatologist at the National Expertise Center for Atopic Dermatitis.
The CAFÉ study enrolled patients who were at minimum 18 years with an Eczema Area and Severity Index (EASI) of equal to or greater than 20 and an Investigator Global Assessment (IGA) of 3 or 4 who had no prior cyclosporine A exposure and either not currently a candidate for cyclosporine treatment or who had previous exposure to cyclosporine A but cyclosporine A treatment could not be restarted due to intolerance, inadequate response or a requirement for doses more than 5 mg/kg/day or a duration more than one year. They were randomized to dupilumab, 300 mg subcutaneously weekly or every other week, with use of topical corticosteroids or placebo plus topical steroids. A total of 318 patients completed 16 weeks in the study, 108 in the weekly dupilumab arm, 107 in the every other week dupilumab arm, and 103 in the placebo group.
Dr. Weller noted that the EASI score required for inclusion (≥20) was higher than in previous studies of dupilumab. The median EASI score ranged from 31.1 to 31/7 in the 3 arms, indicating moderate to severe disease, and the median Scoring Atopic Dermatitis (SCORAD) score was 66.1 to 67.5. About half the patients in each arm had an IGA =4. About 70% in each arm were treated previously with cyclosporine A. Nearly 60% of patients had a Hospital Anxiety and Depression Scale (HADS)-A or HADS-D at least 8.
A significantly percentage number of patients treated with dupilumab achieved an EASI-75 at week 16, the primary endpoint of the study, compared with placebo. These percentages were 62.8 percent in the group receiving dupilumab every two weeks, 59.1 percent receiving weekly dupilumab, and 29.6 percent in the placebo recipients (P<0.0001). This result was similar in the population with prior cyclosporine A use: 58.0 percent, 56.5 percent, and 26.4 percent, respectively, achieved an EASI-75.
The mean percent change in EASI score from baseline was nearly 80 percent in patients assigned to dupilumab at either dosage compared with 46.6 percent in the placebo group (P<0.0001 for both dupilumab groups vs. placebo). The mean percent change in SCORAD from baseline was 58.3 percent (weekly) and 62.4 percent (every other week) in the dupilumab arms, compared with 29.5 percent in the placebo group (P<0.0001 for both dupilumab groups vs. placebo).
Dupilumab also induced a significantly greater reduction in the weekly average of peak daily pruritus from baseline compared with placebo (P<0.0001 with either dosage vs. placebo at 16 weeks). A significant effect on itch was already observed at week two, Dr. Bruin-Weller said.
A significant improvement in the Patient-Oriented Eczema Measure and the Dermatology Quality of Life Index at week 16 was also seen with both dupilumab dosages vs. placebo (P<0.0001 for both dosages on both outcomes). More than twice as many patients in the dupilumab groups reported no pain or discomfort at week 16 vs. placebo (P<0.0001 in the very other week group vs. placebo; P=0.0004 in the weekly group vs. placebo).
As with pervious trials of dupilumab, conjunctivitis was more frequent with dupilumab-16.4 percent with every other week dosing and 28.0% with weekly dosing-compared with placebo (11.1 percent).
Andrew Bleauvelt, M.D., president, Oregon Medical Research Center, Portland, who was not involved in this study but is an investigator on others, commented in a separate presentation that conjunctivitis “is the only significant side effect of this drug that we’ve seen thus far. You do see this condition develop more commonly with time, so you see that the 52-week numbers are higher than at week 16.” Most cases his practice have been mild to moderate and did not interfere with therapy; only one of about 80 patients he has treated has had to stop therapy because of conjunctivitis. Wetting drops and topical steroids are usually sufficient for management.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.