- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
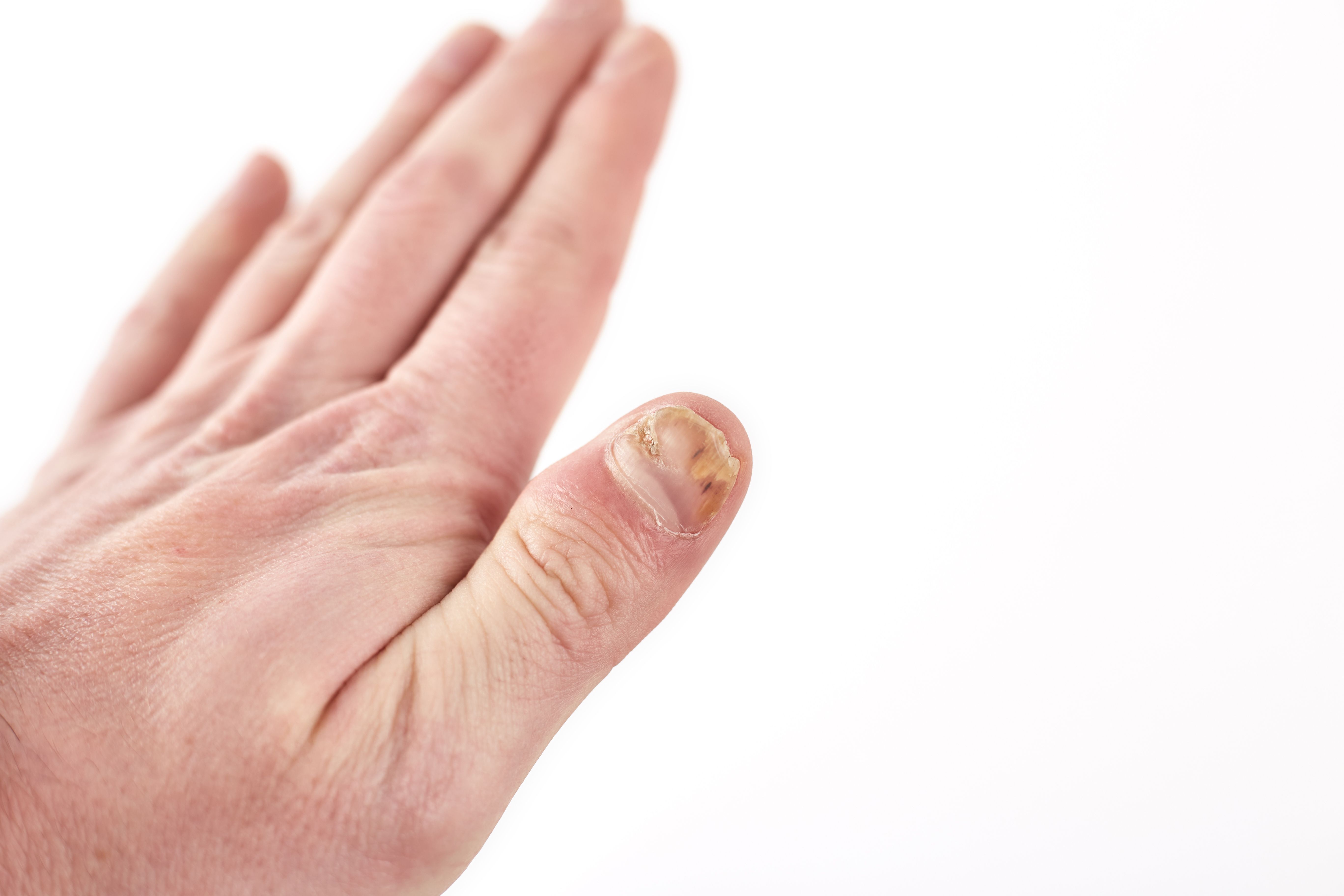
Efinaconazole Demonstrates Superior Transungual Penetration Compared to Other Antifungal Treatments
Author(s):
Topical antifungals, whether FDA-approved or available over-the-counter, exhibited more effective penetration through cellulose disks than cadaver nails.
A recent set of in vitro experiments aimed to compare the penetration efficiency of commercially available topical antifungals through keratin-free cellulose disks versus human nails, shedding light on the potential inhibitory impact of keratin on Trichophyton growth. The research was included in a poster presented at the Maui Derm Hawaii 2024 conference in Wailea, Hawaii.
The study tested 7 topical antifungals treatment including 3 that were approved by the US Food and Drug Administration (FDA) for onychomycosis treatment. These included ciclopirox 8% lacquer, efinaconazole 10% solution, and tavaborole 5% solution.
Additionally, researchers assessed and compared these with 4 over-the-counter (OTC) products for fungal infections, including tolnaftate 1% solutions: Formula 3, Formula 7, and Tolcylen, and undecylenic acid 25% solution (Terpenicol).
Antifungal efficacy was evaluated through a cellulose disk diffusion assay. The impact of nail penetration on antifungal efficacy was assessed by applying the products to human cadaverous toenails.
In the cellulose disk diffusion assay, efinaconazole and tavaborole, both FDA-approved topicals, demonstrated maximal inhibition against T. rubrum and T. mentagrophytes, with a zone of inhibition (ZI) of 85 mm.
Ciclopirox exhibited lower average ZIs (59.0 mm and 55.7 mm, respectively). For OTC products, ZIs ranged from 31.2 to 57.8 mm against T. rubrum and 25.7 to 47.7 mm against T. mentagrophytes.
In the nail penetration assay, efinaconazole showed the greatest average ZI against both species (T. rubrum: 82.1 mm, T. mentagrophytes: 63.8 mm), followed by tavaborole (63.5 mm, 39.1 mm) and ciclopirox (7.4 mm, 3.6 mm).
OTC products exhibited ZIs ranging from 10.5 to 34.2 mm against both species. Nail thickness did not significantly affect ZIs.
The study's findings suggest that FDA-approved and OTC topical antifungals penetrate cellulose disks more efficiently than cadaver nails, according to study authors.
"Among all antifungals tested, ability to penetrate human toenails and inhibit growth of both T. rubrum and T. mentagrophytes was greatest for efinaconazole, followed by tavaborole," wrote study authors Elabbasi et al. "These results indicate superior transungual penetration of efinaconazole compared to the other antifungals, perhaps due to lower keratin binding in the nail."
Reference
Elabbasi A, Eltokhy A, Joseph W, Elewski B, Ghannoum M. Transungual penetration and antifungal activity of seven prescription and over-the-counter topical antifungals: in vitro comparison.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.