- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Enrollment Milestone Achieved in Phase 3 Clinical Trials for DFD-29 Treatment of PPR
Author(s):
Topline data expected in the first half of 2023.
Journey Medical Corporation has announced a 50% enrollment rate for its Phase 3 clinical trials to assess the efficacy of Minocyclinemodified releasecapsules 40 (DFD-92), for treating papulopustular rosacea.1
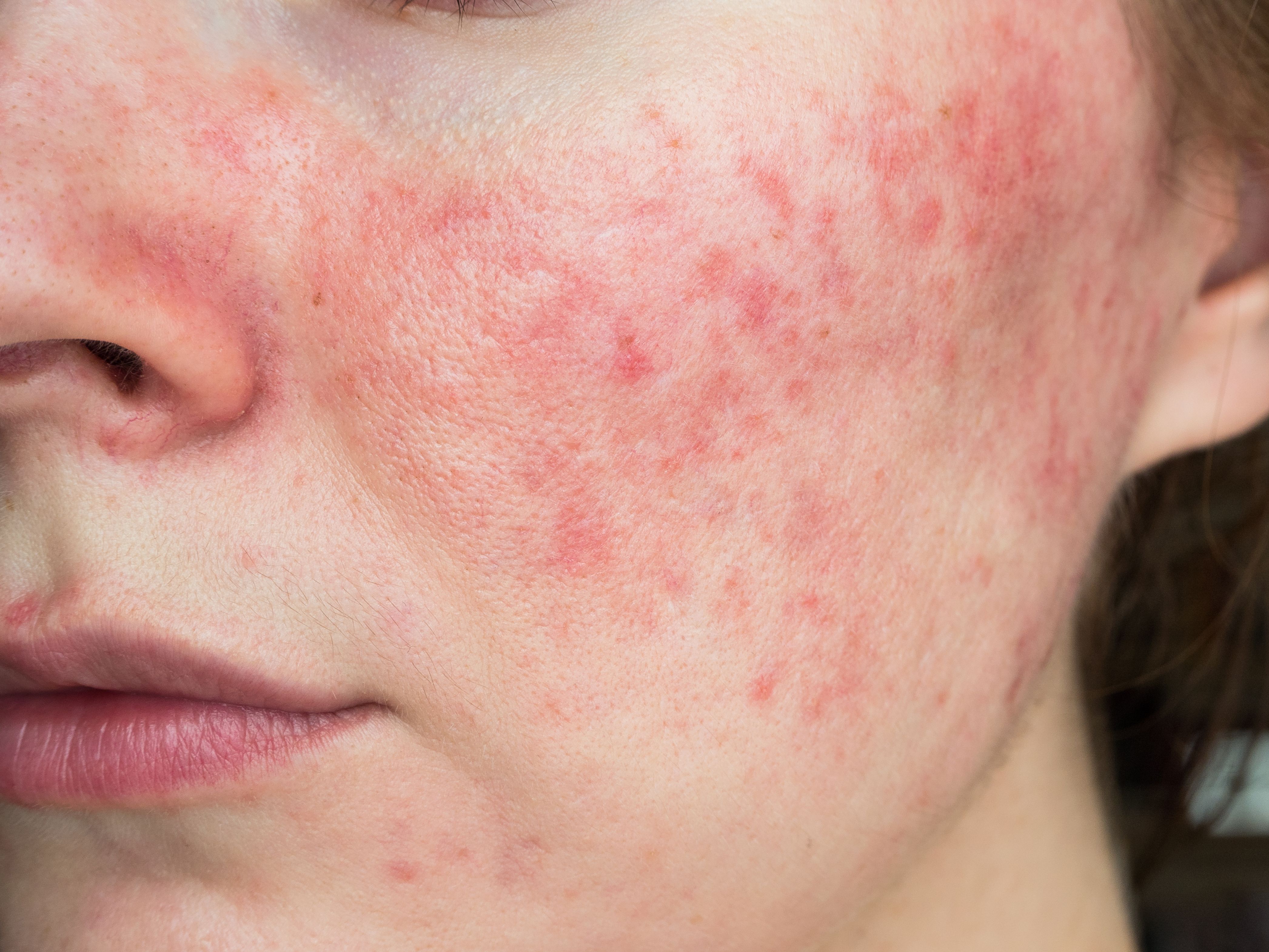
Rosacea is a chronic but treatable condition that primarily affects the central face and is often characterized by flare-ups and acne-like inflammatory lesions (papules and pustules). According to the National Rosacea Society, the condition affects at least 14 million people in America and as many as 415 million worldwide.
Journey Medical Corporation’s cofounder, ClaudeMaraoui, expressed satisfaction with the milestone and applauded the drug’s success so far. The phase 3 trial of DFD-29 consists of 640 adult patients; more than 50% of whomsuffer from papulopustular rosacea. The safety and efficacy of the drug is being compared to placebo treatments, as well asdoxycycline capsules.Doxycycline is currently the only drug approved by the FDA to specifically treat papulopustular rosacea.2
The phase 3 clinical trials are part of a collaboration with India based Reddy’s Laboratories LTD. for the ongoing development and commercialization of the DFD-29 program. To date, no safety issues have been reported, and no drug-related adverse effects have been observed.
Journey Medical plans to announce topline data in the first half of 2023, with a newdrug application expected in the second half of 2023. When approved, DFD-29 could net salesmore than $100 million in revenue.
References:
- Corporation JM. Journey medical corporation announces 50% enrollment milestone achieved in phase 3 clinical trials evaluating dfd-29 (Minocycline modified release capsules 40 mg) for the treatment of papulopustular rosacea. GlobeNewswire Newsroom.
- Goldgar C, Keahey DJ, Houchins J. Treatment options for acne rosacea. afp. 2009;80(5):461-468.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.