- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
EON Gains FDA Clearance for Back and Thighs
Author(s):
The robotic device was previously cleared to treat patients' full abdomen and flanks.
Eon Smarter Body Contouring, developed by Dominion Aesthetics Technologies, Inc., has received clearance from the US Food and Drug Administration (FDA) to treat the back and thighs.1 Eon, a robotic, touchless, 1064nm laser used for non-invasive lipolysis, was previously FDA-cleared to treat patients’ full abdomen and flanks.
According to a press release, EON’s laser heats fat up to 123.8F, causing fat-cell death with natural flushing through the lymphatic system. EON hovers over the body during treatment, memorizing the topography and using sensors to detect skin temperature, room temperature, distance from skin, laser energy, jet temperature, which are part of the device’s safety protocol.
photo credit: Dominion Aesthetic Technologies, Inc.
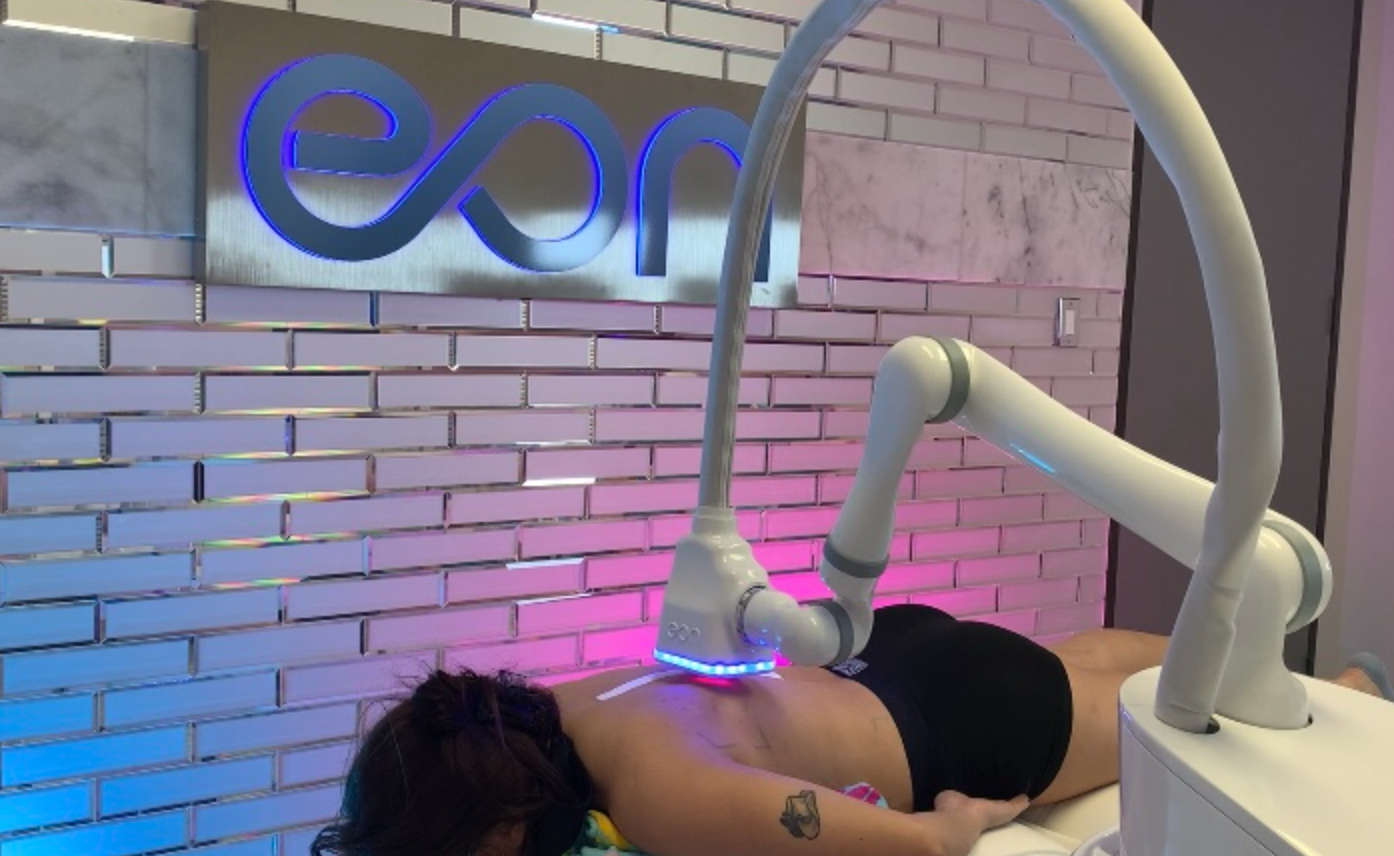
One treatment consists of multiple segments, each running 20 minutes. Flanks can be treated in 40 minutes, and the entire abdomen, in 60 minutes. Dominion stated that EON has “unrivaled efficacy” and does not contain time-consuming gels, applicators, or post-treatment messages, which are typically needed with other body contouring devices.
Thomas Fiala, MD, owner of Fiala Aesthetics in Orlando, Florida, led a small-scale week study in 2019 to examine the efficacy of the EON robotic device. 26 patients (20 females/6 males) ages 21 to 61, and with a body mass index of 17.8 to 32.3 were underwent body contouring using the device. 2
After a single usage, Fiala reported:
- Ultrasound average of fat reduction was 21.6% (25.3% in lower abdomen)
- Average circumferential reduction was 4.1cm/1.6 in
- Average fat reduction was 6.3 mm.
- Over 73% of patients showed greater than 20% fat reduction.
Dominion Chief Executive Officer Cooper Collins said, “With our new FDA clearance, EON is proving, while using state-of-the-art technology, that we can adapt to any environment to benefit all patients and offer providers maximum flexibility. We will continue to improve our device and its uses to ensure that EON keeps its place as the premium device for body contouring.”
In 2021 Dermatology Times® featured a full review of EON with Michael Kluska, DO, and Chad Deal, MD, of Southern Surgical Arts, Chattanooga, Tennessee, including device specifics, cost, ROI and more.
Eon is available through a network of medical providers across the US, and can be accessed at www.eonlaser.com.
To find out what else is new, trending and up-and-coming in the world of laser therapy in dermatology, visit Dermatology Times.
References
1. EON Gains FDA Clearance for Back and Thighs. Dominion Aesthetic. March 2023. https://dominionaesthetic.com/eon-gains-fda-clearance-for-back-and-thighs/. Accessed March 29, 2023.
2. Unique Benefits of EON for Doctors. EONLASER. https://www.eonlaser.com/unique-benefits-of-eon-for-doctors/. Published December 23, 2020. Accessed March 29, 2023.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














