- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
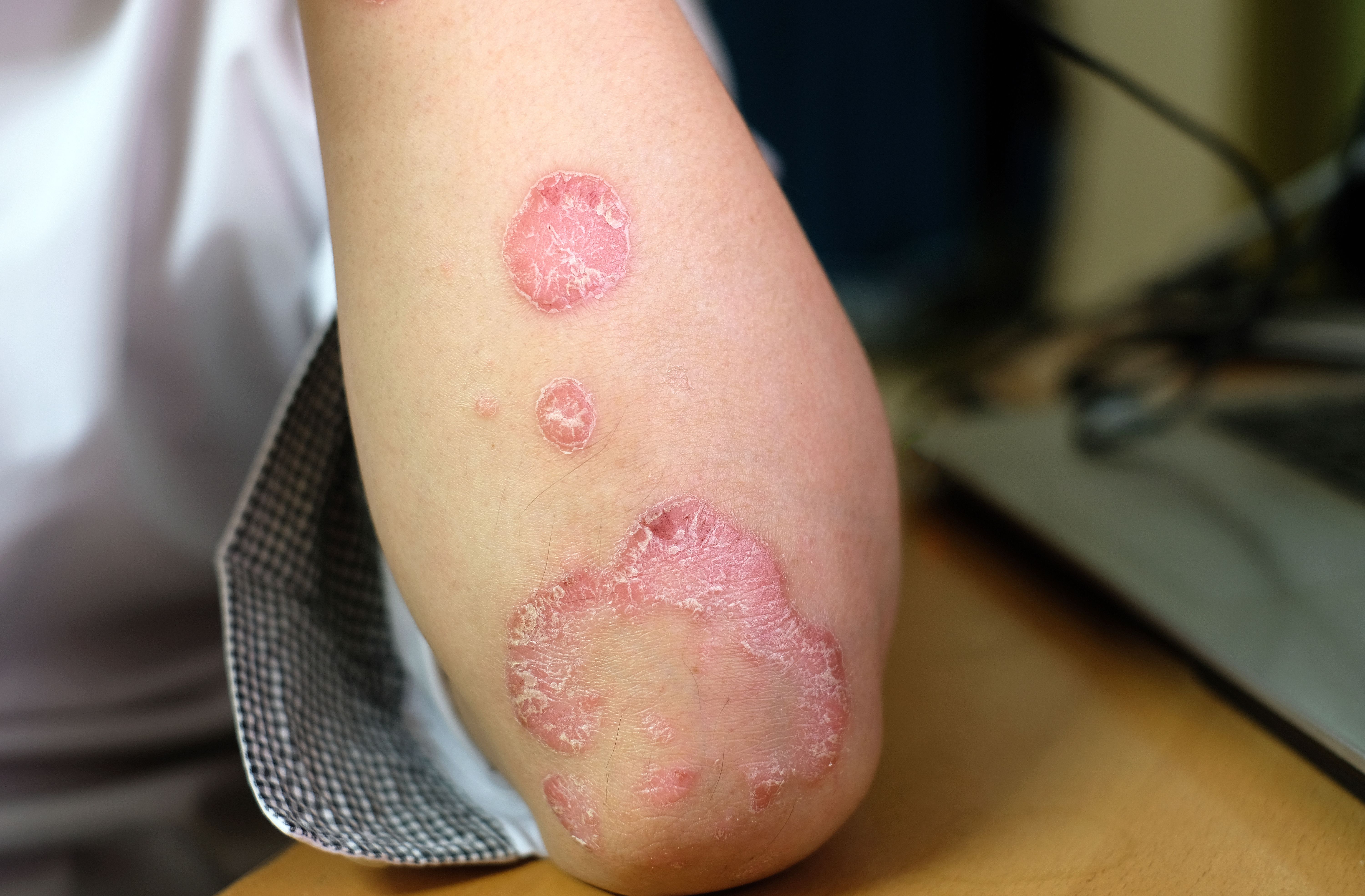
FDA Approves Lebrikizumab-lbkz for Moderate to Severe Atopic Dermatitis
Ebglyss is now approved for children and adults aged 12 years and older.
The US Food and Drug Administration (FDA) has approved Eli Lilly and Company's lebrikizumab-lbkz (Ebglyss) for children and adults aged 12 years and older with moderate to severe atopic dermatitis (AD).1
The IL-13 inhibitor is intended for adults and pediatric patients whose AD has not yet been controlled through the use of topical therapies. Children 12 years of age and older must weigh at least 88 pounds (40 kg).
Image credit: © Calin - stock.adobe.com

The approval is supported by positive data stemming from the ADvocate 1, ADvocate 2, and ADhere studies.
"Patients still struggle to control their moderate-to-severe atopic dermatitis with currently available therapies. Many experience poor long-term disease control, and severe itch can significantly impact their daily lives," said Jonathan Silverberg, MD, PhD, MPH, professor of dermatology at George Washington University School of Medicine and Health Sciences in Washington, DC, in a news release.1 Silverberg is also the first author of the manuscript for the clinical trials related to lebrikizumab.
The studies, involving over 1,000 patients with moderate to severe eczema who didn't respond to topical treatments, primarily measured clear or almost clear skin at the 16 week mark.
In an average of the ADvocate 1 and ADvocate 2 studies, 38% of those using lebrikizumab achieved clear skin at 16 weeks, compared to 12% with placebo, and 10% saw results as early as 4 weeks. Of those who achieved clear skin, 77% maintained it for a year with monthly doses, and 48% maintained it even after switching to placebo.
For itch relief, 43% of lebrikizumab users experienced relief at 16 weeks, versus 12% with placebo, with 5% feeling relief as early as 2 weeks. Among those with itch relief at week 16, 85% continued to feel relief after a year on monthly maintenance, and 66% maintained relief after switching to placebo.
"Today's FDA approval of Ebglyss is a big win for patients, as we now have a new first-line biologic treatment option for moderate-to-severe disease when topical prescriptions aren't enough," he said.
Kristin Belleson, president and CEO of the National Eczema Association, weighed in on the approval's significance for patients.
"Eczema can affect people of all skin tones, ethnicities, genders and ages. Nearly 16.5 million adults in the US have eczema, with 6.6 million experiencing moderate-to-severe symptoms like itchiness, dry and scaly skin, discoloration and rashes, which can lead to more scratching that may cause skin to crack and bleed," Belleson said. "The approval of Ebglyss provides hope and promise for the eczema community and those still seeking lasting relief from disruptive symptoms."
Lebrikizumab was approved by the European Commission in 2023,2 Japan in January 2024, and Canada in June 2024.3
Lebrikizumab will be available as a 250 mg/2 mL injection and may be used with or without topical corticosteroids. Lilly anticipates the drug will become available in the US in a matter of weeks.
References
- FDA approves Lilly's Ebglyss (lebrikizumab-lbkz) for adults and children 12 years and older with moderate-to-severe atopic dermatitis. News release. Lilly. September 13, 2024. Accessed September 13, 2024. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-ebglysstm-lebrikizumab-lbkz-adults-and#:~:text=INDIANAPOLIS%20%2C%20Sept.%2013%2C%202024,88%20pounds%20(40%20kg)%20with
- Almirall. Almirall receives European Commission approval of Ebglyss (lebrikizumab) for moderate-to-severe atopic dermatitis. News release. November 17, 2023. Accessed September 13, 2024. https://www.almirall.com/newsroom/news/almirall-receives-european-commission-approval-of-ebglyss-lebrikizumab-for-moderate-to-severe-atopic-dermatitis
- Health Canada authorizes Lilly's Ebglyss (lebrikizumab) for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years and older. News release. Cision; Eli Lilly Canada Inc. June 25, 2024. Accessed September 13, 2024. https://www.newswire.ca/news-releases/health-canada-authorizes-lilly-s-ebglyss-tm-lebrikizumab-for-the-treatment-of-moderate-to-severe-atopic-dermatitis-in-adults-and-adolescents-12-years-and-older-833722064.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.