- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
FDA Approves Topical Gel Filsuvez for Epidermolysis Bullosa
Author(s):
Chiesi Global Rare Diseases recently announced the approval of the topical treatment.
tibanna79/Adobe Stock
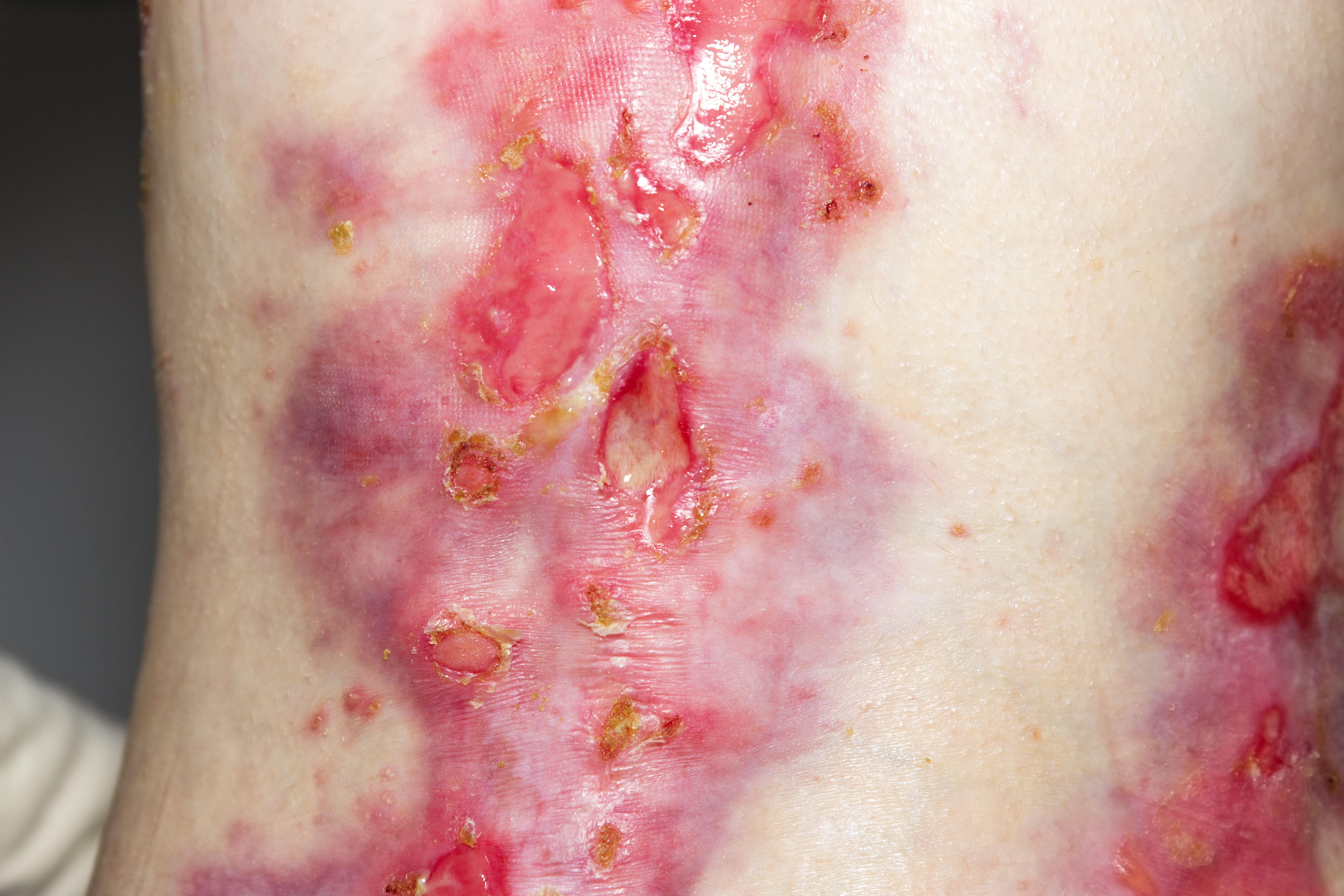
Chiesi Global Rare Disease recently announced the US Food and Drug Administration's (FDA) approval of Filsuvez (birch triterpenes) topical gel for the treatment of partial thickness wounds related to dystrophic junctional and epidermolysis bullosa (DEB; JEB) in patients 6 months and older.
The approval makes Filsuvez the first approved treatment for patients with wounds associated with JEB, a more rare and often severe form of EB that often begins with blisters notable in infancy.
In July 2022, the European Commission approved FILSUVEZ for adults and children as young as 6 months of age exhibiting skin wounds related to DEB and JEB.
The European Commission approval was based on the EASE trial (NCT03068780). EASE was the largest global phase 3 trial conducted in patients with EB.
Conducted across 58 sites spanning 28 countries, the study involved a 3-month double-blind randomized controlled phase, followed by a 24-month open-label, single-arm phase. Participants with DEB and JEB were included if their target wounds measured between 10 and 50 cm2, persisted for more than 21 days but less than 9 months.
During the double-blind phase, patients were randomly assigned to receive the study treatment or follow standard wound care, maintaining a 1:1 ratio. A total of 223 participants, including 156 pediatric patients, were enrolled in the trial. All individuals who completed the double-blind phase transitioned to the open-label safety follow-up phase.
“The FDA’s decision to approve FILSUVEZ provides those living with EB a safe and effective treatment option for the most prominent and difficult symptom of EB, open wounds that may not heal," said Brett Kopelan, Executive Director, debra of America, in a press release. "Today marks an important milestone for those living with junctional EB, as FILSUVEZ is the first FDA approved treatment for this variant of the disease. I want to thank Chiesi for their years of close collaboration with debra of America and their dedication and commitment to bringing a treatment option forward for those with dystrophic and junctional EB. I also want to thank the patients who participated in the clinical trials to bring this therapeutic option to fruition.”
Filsuvez will be available for patients to administer in an at-home setting and is administered directly to the wound at each dressing change.
Reference
Chiesi Global Rare Diseases. Chiesi Global Rare Diseases receives FDA approval for FILSUVEZ (birch triterpenes) topical gel for the treatment of epidermolysis bullosa. GlobeNewswire News Room. December 19, 2023. Accessed December 19, 2023. https://www.globenewswire.com/news-release/2023/12/19/2798751/0/en/Chiesi-Global-Rare-Diseases-Receives-FDA-Approval-for-FILSUVEZ-birch-triterpenes-topical-gel-for-the-Treatment-of-Epidermolysis-Bullosa.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.















