- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
FDA Clears Brella SweatControl Patch for Reduced Primary Axillary Hyperhidrosis
Author(s):
Patients can achieve reduced excessive underarm sweating after one in-office treatment.
Photo courtesy of Candesant Biomedical

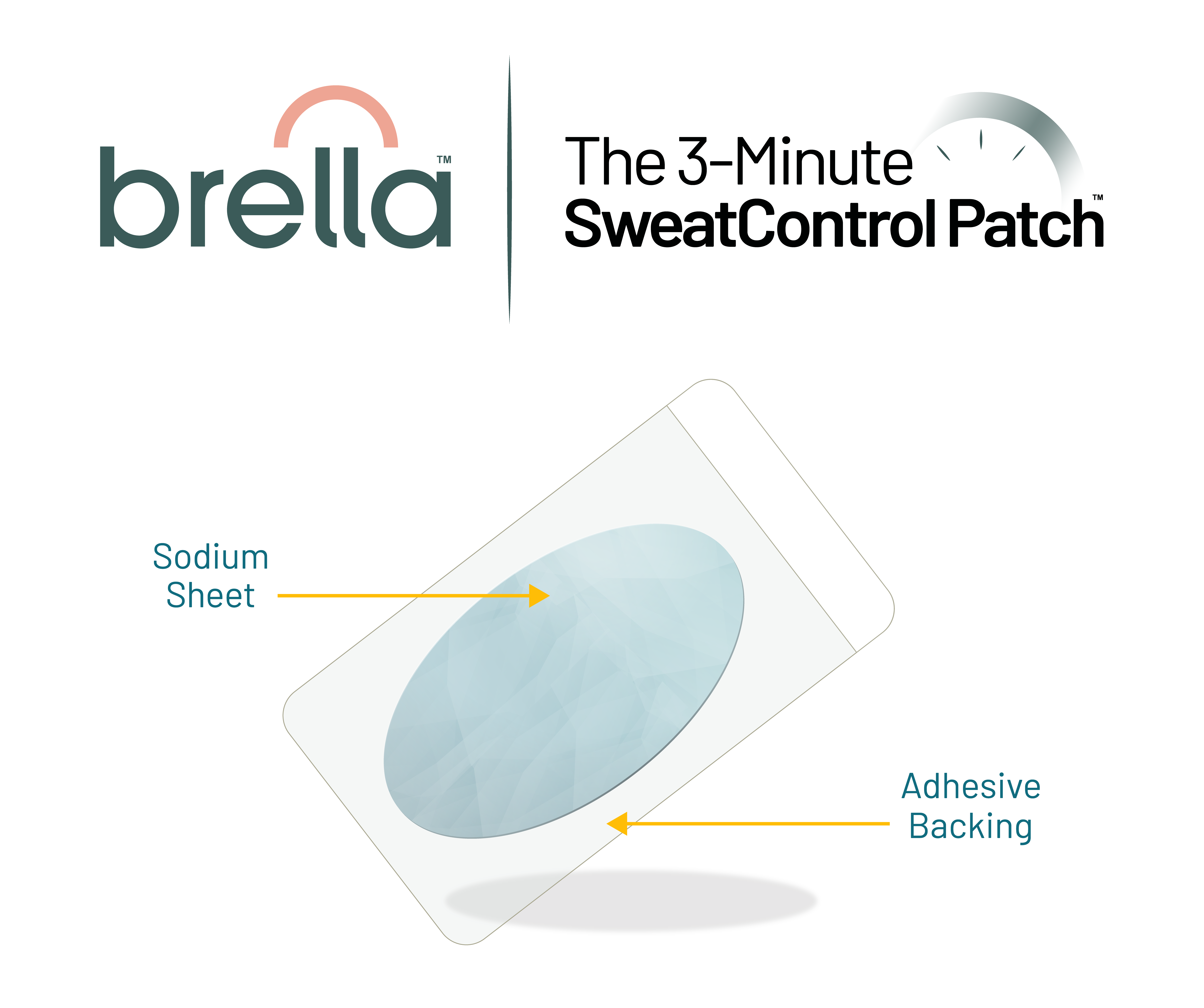
The US Food and Drug Administration (FDA) has cleared Candesant Biomedical’s Brella, 3-Minute SweatControl Patch for the treatment of adult patients with primary axillary hyperhidrosis.1 Brella uses Candesant’s patented targeted alkali thermolysis (TAT) technology to reduce excessive underarm sweating as well as negative impacts associated with excessive sweating during daily activities. The in-office treatment is non-invasive, needle-free, and aluminum-free, and provides patients with results lasting 3 to 4 months.
Brella is a single-use disposable sodium patch applied to a patient’s underarm for approximately 3 minutes. Candesant’s TAT technology uses the scientific principle of heat is generated when sodium comes into contact with water in sweat. When the sodium sheet is applied to the underarm, the thermal energy created is precisely localized to microtarget sweat glands and reduces overall sweat production. According to Candesant, Brella is the first clinical application to utilize the reaction between sodium and sweat and is the only patented medical device based on the TAT technology.
The FDA’s clearance of Brella is based on data from SAHARA (NCT04599907), a randomized, double-blind, sham-controlled, multicenter pivotal study that included 110 adult patients with primary axillary hyperhidrosis. Patients had baseline Hyperhidrosis Disease Severity Scale (HDSS) scores of 3 or 4, which showed that excessive sweating frequently or always interfered with their daily lives. Patients then received bilateral treatment with Brella or a sham patch for up to 3 minutes on each underarm. Afterward, patients were evaluated weekly for 12 weeks post-treatment, with responders followed for up to 24 weeks post-treatment.
Photo courtesy of Candesant Biomedical
Primary endpoints of the SAHARA study included the occurrence of adverse events and skin reactions following patch treatment (2-week time frame) and achievement of an HDSS score of 1 or 2 (4-week time frame). Key secondary endpoints included a mean improvement in quality of life and the proportion of treated subjects with at least 50% improvement in Gravimetric Sweat Production (GSP) from baseline to 4 weeks. Eligible patients were aged 22 years or older with GSP greater than 50mg per axilla.2
Brella was well tolerated among patients with no reported serious or severe adverse events. Data from the SAHARA study was presented in a late-breaking oral presentation at the 2023 American Academy of Dermatology Annual Meeting in New Orleans, Louisiana.
Photo courtesy of Candesant Biomedical

Brella will be available in certain US marketing starting in late summer of 2023 through the Candesant Brella Early Experience Program which will include health care providers with aesthetic practices with experience treating patients with excessive sweating. Brella will be launched nationally following the Brella Early Experience Program.
References
- Candesant Biomedical receives FDA clearance of Brella, the first and only 3-minute sweatcontrol patch to significantly reduce primary axillary hyperhidrosis. Candesant Biomedical. Published April 13, 2023. Accessed April 13, 2023. https://www.multivu.com/players/English/9159151-candesant-biomedical-receives-fda-clearance-of-brella/
- Evaluation of N-SWEAT patch for treatment of primary axillary hyperhidrosis or excessive axillary sweating. ClinicalTrials.gov identifier: NCT04599907. Updated August 2, 2021. Accessed April 13, 2023. https://clinicaltrials.gov/ct2/show/NCT04599907?term=SAHARA%2C+hyperhidrosis&draw=2&rank=1
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














