- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
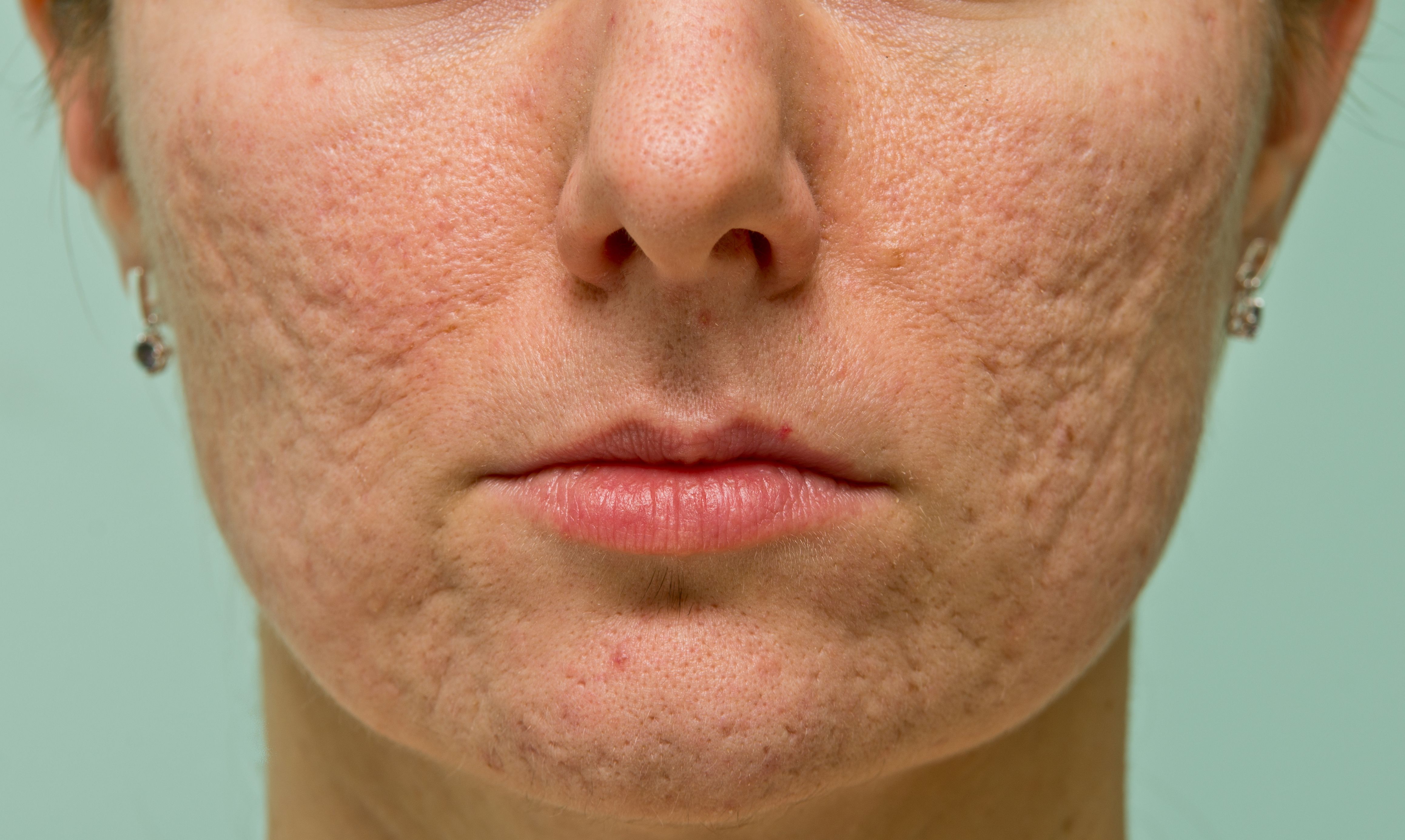
FDA Clears Sofwave SUPERB Applicator for the Treatment of Acne Scars
Author(s):
From its clinical study, 97% of patients saw improvement in acne scars after 3 treatment sessions.
Sofwave Medical Ltd announced the FDA clearance of its Precise SUPERB Applicator for the treatment of acne scars. The recent approval adds an additional treatment indication to Sofwave’s SUPERB device, which now includes improving acne scars, facial lines and wrinkles, neck tissue, cellulite, and lifting the eyebrows.1
Sofwave conducted a multicenter clinical study to evaluate the efficacy of the SUPERB system for the improvement in the appearance of acne scars. In total, 67 patients were treated with SUPERB across 4 US locations. After 3 sessions, 97% of patients showed improvement in the appearance of acne scars. The study showed a mean improvement level of 1.05±0.53 units based on the acne scar severity scale, indicating a 46% improvement relative to the average baseline acne scar severity grading.
Overall, 88% of patients reported improvement in their acne scar appearance and were highly satisfied with their results. Additionally, the clinical study demonstrated a favorable safety profile, and no serious adverse events were reported during the study.
“Post-acne scarring has a high prevalence and is typically a result of active acne during adolescence and younger age,” said Louis Scafuri, CEO of Sofwave Medical, in the news release. “Gaining FDA clearance to market SUPERB for the treatment of acne scarring not only paves the way to positively impact patients seeking improved appearances but also in situations where the presence of post-acne scarring has negatively impacted an individual’s perceived body image, self-esteem, and mental health. Further, we will mirror our efforts to bring this new acne scarring treatment to the U.S. with our other key territories while continuing to expand regulatory approvals for additional medical aesthetic treatment indications. Present energy based treatment regimens are limited due to the risk of post inflammatory hyperpigmentation associated with other energy based treatments.”
Previous Indications
Recently, the SUPERB Applicator was cleared by the FDA in March 2023 for the treatment of improving facial lines and wrinkles. In December 2022, the FDA cleared Sofwave’s device for the short-term treatment of cellulite. When evaluating the efficacy of SUPERB’s system for cellulite, blinded reviewers were able to correctly identify the post-treatment images for 89% of the patients.
Reference
- Sofwave Medical announced FDA clearance of SUPERB technology for treatment of acne scars. August 30, 2023. Accessed August 30, 2023. https://www.biospace.com/article/releases/sofwave-medical-announces-fda-clearance-of-superb-technology-for-treatment-of-acne-scars/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.