- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
FDA Clears Sofwave to Improve Appearance of Cellulite
Author(s):
The non-invasive treatment has no downtime for patients.
SofwaveTM now offers patients a US Food and Drug Administration (FDA) cleared treatment to reduce the appearance of cellulite by targeting and thickening the dermal layer1. The procedure relies on a novel delivery method of ultrasound technology called synchronous ultrasound parallel beam technology (SUPERBTM) to stimulate collagen formation. The treatment is safe for all skin types and colors.
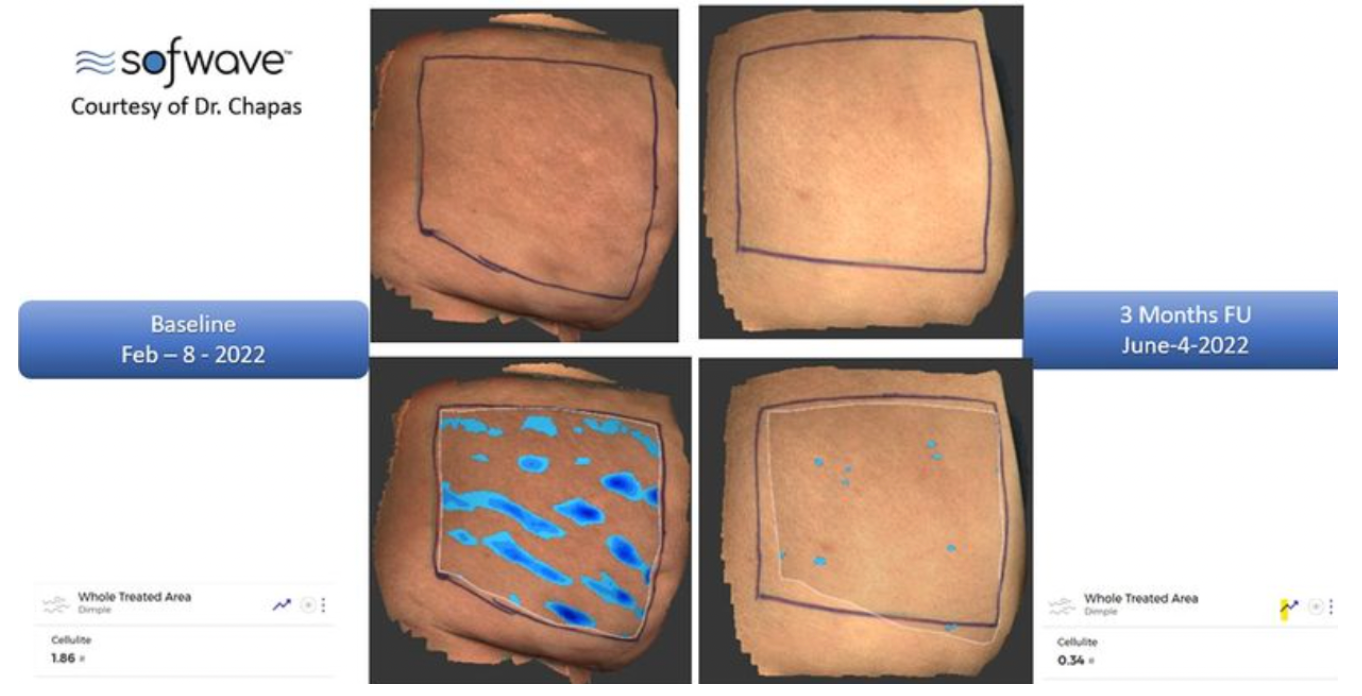
A single 30–45-minute session was previously known for softening wrinkles and supporting lax skin around a patient’s eyebrows, skin, and neck. The new FDA clearance means Sofwave is clinically proven to reduce the appearance of cellulite to give patients more tout skin on the thighs and buttocks.
A clinical study of Sofwave provided 2 treatments spaced out 2-4 weeks apart on the upper thighs and buttocks of study participants (N=68). Photographs taken at baseline and the 3-month post-procedure mark were assessed by blinded independent reviewers to identify and grade pre-treatment and post-treatment images. Reviewers correctly identified the post-treatment images for nearly 90% of study participants. Improvement was also evaluated using the Global Aesthetic Improvement scale (GAIS) and Laxity Scale (LS). No serious or unanticipated adverse effects were reported during the study.
The global cellulite treatment market in 2021 was valued at more than $1 billion and expected to grow at a compound annual growth rate of 11% through 20302.
References
1. Eckhouse DS, Scafuri L. Sofwave medical announces FDA clearance of SUPERB™ Technology for cellulite. Sofwave. https://sofwave.com/news/sofwave-medical-announces-fda-clearance-of-superb-technology-for-cellulite/. Published January 9, 2023.Accessed January 12, 2023.
2. Grandview Research. Cellulite treatment market size & share report, 2022-2030. Cellulite Treatment Market Size & Share Report, 2022-2030. https://www.grandviewresearch.com/industry-analysis/cellulite-treatment-market. Published August 9, 2022. Accessed January 12, 2023.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











