- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
FDA Grants Priority Review of Abeona’s Pz-cel BLA for Recessive DEB
Author(s):
The FDA’s PDUFA target date is May 25, 2024.
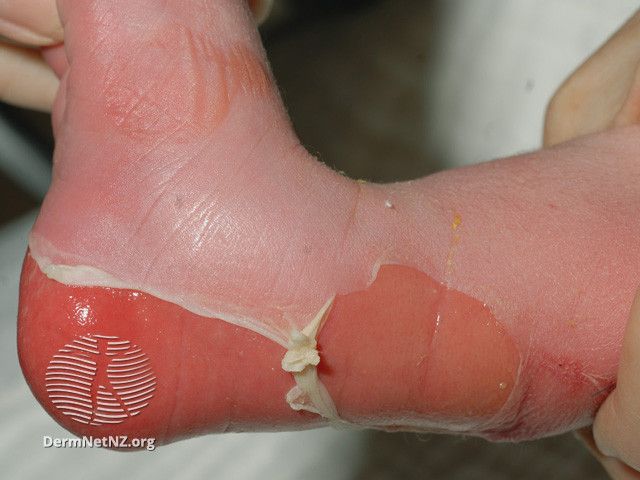
Abeona Therapeutics recently announced that the US Food and Drug Administration (FDA) has accepted and granted Priority Review for its biologic license application (BLA) for pz-cel (prademagene zamikeracel), investigational autologous, COL7A1 gene-corrected epidermal sheets for the treatment of patients with recessive dystrophic epidermolysis bullosa (RDEB). The FDA’s PUDFA target date for pz-cel is May 24, 2024. According to the announcement, the FDA "advised that it does not currently plan to convene an Advisory Committee meeting to discuss the pz-cel application.”
The FDA’s acceptance of the BLA is based on the clinical efficacy and safety data from the pivotal phase 3 VIITAL study (NCT04227106) and additional confirmatory data from the phase 1/2a study (NCT01263379). Both studies demonstrated that a one-time application of pz-cel on large and chronic wounds delivered sustained wound healing and pain reduction for patients with RDEB. According to Abeona, the FDA’s grant of priority review status is an important prerequisite for Abeona’s eligibility for a Priority Review Voucher.
“The FDA’s acceptance of our BLA for priority review underscores the high unmet need in RDEB and the potential for pz-cel to provide meaningful benefit to these patients,” said Vish Seshadri, the chief executive officer of Abeona, in the news release. “We thank the FDA for their commitment and look forward to working with them through the BLA review, with the goal of bringing this therapy to patients as soon as possible.”
Pz-cel is designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells and enable long-term gene expression by using a retroviral vector to stably integrate into the dividing target cell genome. Additionally, pz-cel is being investigated for its ability to enable normal type VII collagen expression and to facilitate wound healing and pain reduction in difficult-to-treat RDEB wounds after a one-time application procedure.
The pivotal phase 3 VIITAL study is a randomized clinical trial that evaluated the efficacy, safety, and tolerability of pz-cel in 43 large chronic wound pairs in 11 patients with RDEB. Pz-cel has been granted Regenerative Medicine Advanced Therapy, Breakthrough Therapy, Orphan Drug, and Rare Pediatric Disease designations by the FDA.
Abeona first submitted their BLA in September of this year based on VIITAL data that showed 81.4% of patients randomized to receivedpz-cel achieved a 50% or greater level of wound healing, and the average reduction in pain from baseline to conclusion was greater among patients treated with pz-cel.
Reference
Abeona Therapeutics announces FDA accepts and grants Priority Review for pz-cel biologics license application (BLA). Abeona Therapeutics. News release. November 27, 2023. Accessed November 28, 2023. https://investors.abeonatherapeutics.com/press-releases/detail/268/abeona-therapeutics-announces-fda-accepts-and-grants
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.