- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
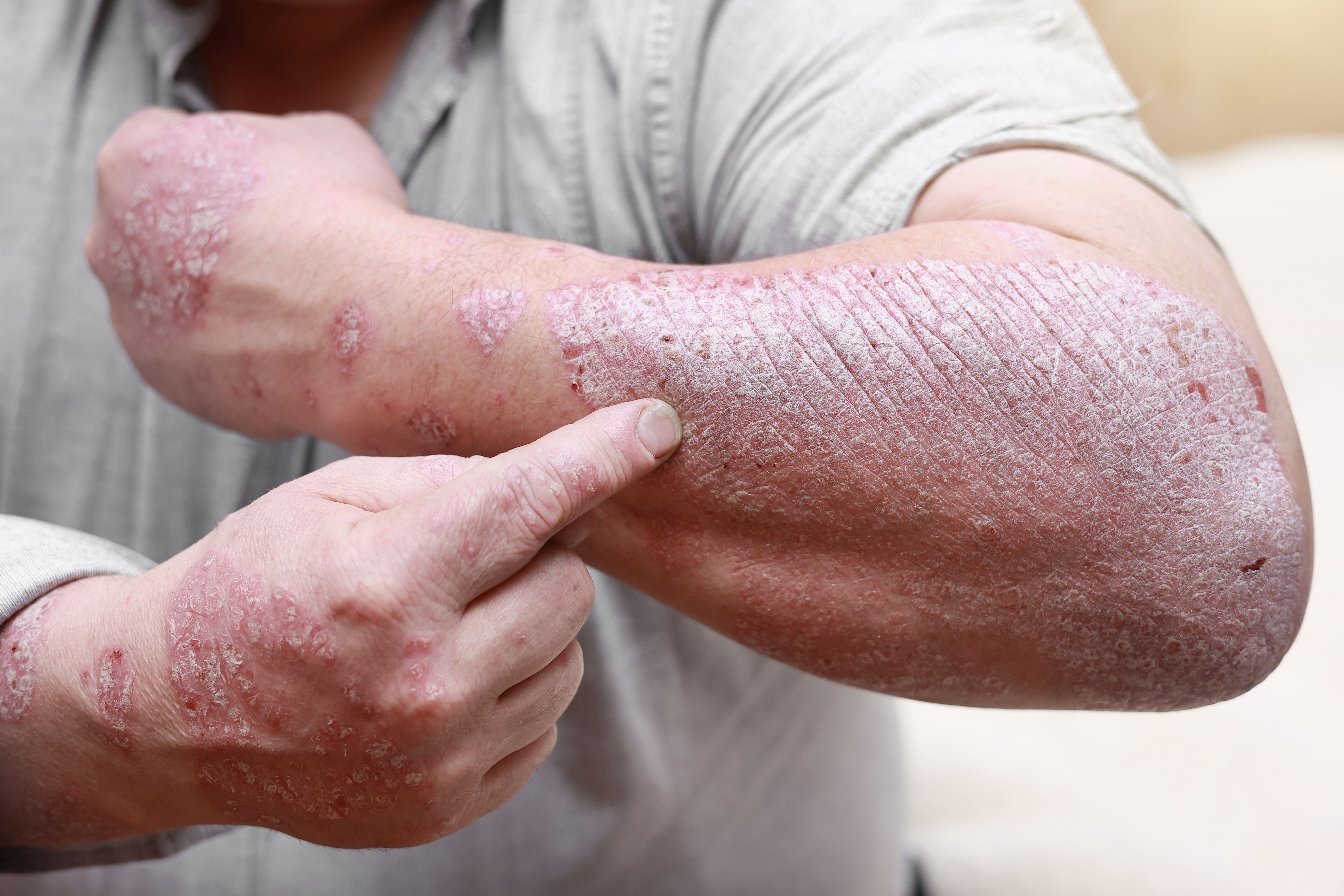
First Patient Dosed in Phase 2a Trial of AX-158 for Psoriasis
Author(s):
Artax announced that safety and efficacy data is expected in the second half of the year.
Artax announced today that the first patient has been dosed in its phase 2a trial (NCT05725057) evaluating the safety and biomarker responses of AX-158 in patients with mild to moderate plaque psoriasis.1
"We are excited about AX-158's potential as the first immunomodulator in the Nck blocker class. Our pre-clinical data supports the potential for AX-158 to realize effective outcomes without the immunosuppression and the side effects associated with existing autoimmune disease therapies," said Artax Chief Executive Officer, Rob Armstrong, PhD, in the news release.1 "We are eager to see this validation when the results from this psoriasis Phase 2a trial are available later this year."
The investigational oral, small molecule immunomodulator selectively modulates T cell responses and acts as a T cell receptor. It has the capability to be used as monotherapy or in combination with another therapy.
The randomized, double-blind, placebo-controlled study will include 30 patients between the ages of 18 and 60 years of age with a minimum diagnosis period of at least 3 months from the time of initial screening.
In this study, patients will be assigned to receive treatment with AX-158 or a placebo during a 28-day treatment period. All patients will be followed and assessed for a 30-day safety period upon the conclusion of treatment.
"The new Nck blocker class of agents, AX-158, has the potential to change how we treat autoimmune disease through targeting and tempering TCR responses so the body only, and properly, reacts against strong pathogens," said James G. Krueger, MD, PhD, head of the laboratory for Investigative Dermatology at The Rockefeller University and Artax Scientific Advisory Board member, in the news release.1 "I am excited to see how this new mechanism of action translates into biomarker changes and patient responses, where to date only targeted immunosuppression has been applied."
Late last year, Artax announced it had been granted clinical trial authorization from the United Kingdom's Medicines and Healthcare Products Regulatory Agency for the phase 2a study.2
"As a physician and as a researcher treating psoriasis for decades, I can attest to the dire need for safe, effective, and tolerable therapies that will truly help patients manage their disease," said Prof. Menno de Rie, MD, of the Department of Dermatology at Amsterdam UMC and an Artax Scientific Advisory Board member, at the time of the clinical trial authorization.2
"I am excited about the innovation and tremendous science AX-158 represents for the many people with psoriasis," de Rie said. "Managing autoimmune disease without inducing the profound immunosuppression associated with current conventional or biological therapies will be transformative for the immunology field."
References
- Artax announces first patient dosed in phase 2a psoriasis trial evaluating AX-158. News release. PR Newswire. February 13, 2024. Accessed February 13, 2024. https://www.prnewswire.com/news-releases/artax-announces-first-patient-dosed-in-phase-2a-psoriasis-trial-evaluating-ax-158-302060157.html
- Artax granted clinical trial authorization for AX-158 phase 2a psoriasis study. News release. PR Newswire. November 9, 2023. Accessed February 13, 2024. https://www.prnewswire.com/news-releases/artax-granted-clinical-trial-authorization-for-ax-158-phase-2a-psoriasis-study-301982709.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.