- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Guselkumab Significantly Clears Scalp Psoriasis in Patients With Skin of Color in Phase 3b VISIBLE
Author(s):
The VISIBLE clinical program will create a longitudinal collection of more than 20,000 clinical images across all skin tones.
MichaelVi/AdobeStock

Johnson & Johnson recently announced new topline data from cohort B of the phase 3b VISIBLE study (NCT05272150) which found guselkumab (Tremfya) demonstrated rapid and significant clearance of moderate to severe scalp psoriasis, as well as significant improvement in scalp itch and patient-reported health-related quality of life (HRQoL) outcomes, including post-inflammatory pigmentation at 16 weeks. The new VISIBLE data was presented at the Maui Derm Hawaii 2024 Conference held January 22 to 26 in Maui, Hawaii.1
VISIBLE is the first prospective, large-scale, randomized-controlled trial dedicated to patients with skin of color across all skin tones with moderate to severe plaque psoriasis and scalp psoriasis to objectively measure clearance and other treatment outcomes with guselkumab. Additionally, the VISIBLE clinical program will create a longitudinal collection of more than 20,000 clinical images across all skin tones to support patients and clinicians with disease recognition. VISIBLE’s cohort B enrolled 108 patients with skin of color with scalp predominant moderate-to-severe psoriasis. According to Johnson & Johnson, scalp psoriasis is a clinically proven difficult-to-treat area. TREMFYA demonstrated significant and rapid scalp psoriasis clearance and improvement in scalp itch as well as patient-reported health-related quality of life outcomes.
“Some of the challenges faced by a study like VISIBLE include the nuances of performing investigator assessments such as PASI and IGA in patients with richly pigmented skin, among whom rating of erythema can be more challenging due to the optical effects of increased melanin pigment. This challenge was overcome by robust investigator training modules and use of confirmatory review of photographs with both standard and polarized lighting,” said Andrew Alexis, MD, MPH, professor of clinical dermatology and vice chair for diversity and inclusion at Weill Cornell Medicine in New York, New York, and VISIBLE’s lead investigator, in a previous interview with Dermatology Times. “Another challenge overcome by the VISIBLE study was the enrollment of patient populations that have historically faced access barriers to clinical trials. This was addressed by broadening the range of study sites and investigators to ensure geographic and demographic diversity.”
Three posters were shared as part of Johnson & Johnson’s guselkumab announcement, including 16-week data on scalp clearance, HRQoL improvements, and patient-reported post-inflammatory pigmentation.
Significant and Rapid Scalp Clearance
Patient with scalp psoriasis before treatment with guselkumab
Image courtesy of Johnson & Johnson
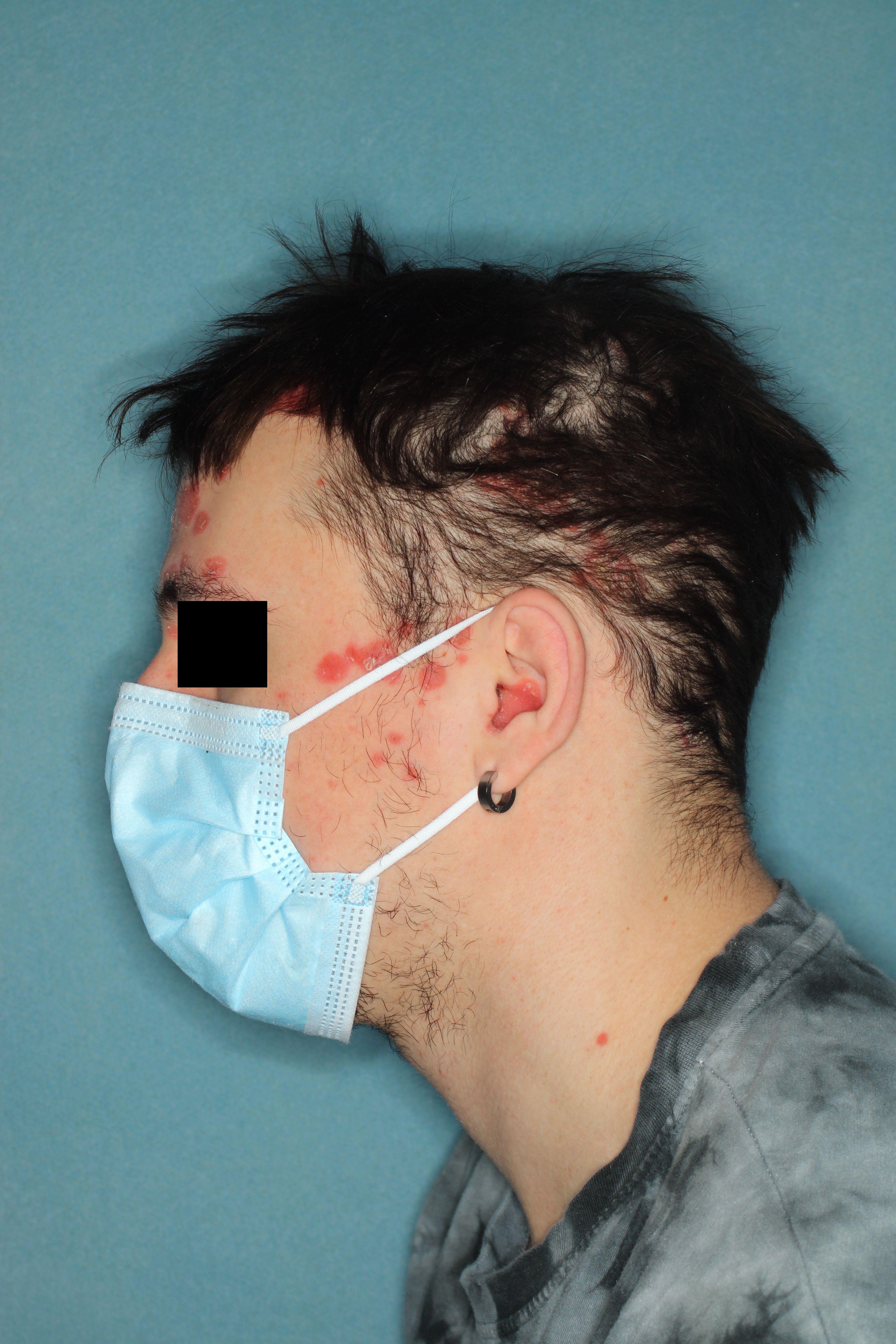
At week 16, after 3 doses of guselkumab, patients with scalp psoriasis achieved almost a 90% improvement from baseline in the Psoriasis Scalp Severity Index (PSSI: 65.8% vs 3.8% placebo) and in Scalp Surface Area (SSA: 86.6% vs 33.4% placebo). Complete scalp clearance was also achieved in the majority of patients who received guselkumab(PSSI 100: 59.2% vs 3.8% placebo; ss-IGA 0: 57.9% vs 3.8% placebo). The median time to achieve PSSI 90 was 11.6 weeks for the guselkumab group and was not achieved by the placebo group. No new safety signals were identified through week 16.2
HRQoL Improvements
At week 16, almost 7 in 10 patients who received guselkumab (69.4% vs 24% placebo) achieved a clinically meaningful improvement on the Scalp Itch Numeric Rating Scale. Also at week 16, patients across all skin tones who received guselkumab reported significantly greater improvements in the Psoriasis Symptoms and Signs Diary and Dermatology Life Quality Index (DLQI). Patients treated with guselkumab also achieved significantly greater mean change from baseline in DLQI compared to placebo-treated participants (-9.7 vs -2.2).3
Patient-Reported Post-Inflammatory Pigmentation
Post-inflammatory pigment alteration (PIPA) can affect all skin tones; however, it can significantly impact the quality of life of patients with skin of color. At baseline, the mean score as assessed by the Skin Discoloration Impact Evaluation Questionnaire (SDIEQ) showed patients experienced at least moderate or greater impact of skin discoloration on their HRQoL. Additionally, at week 16, patients treated with guselkumab reported that they had experienced improvements, with only a mild effect from skin discoloration on their HRQoL.4
According to the announcement, cohort A of the VISIBLE study focused on patients with skin of color with skin predominant psoriasis and provided additional data on the safety and effectiveness of guselkumab across all skin tones. Combined with the latest cohort B results, “the collective data reinforces that the unique study design of VISIBLE is a successful model to enroll, retain, and support people of color who have historically been undertreated and underrepresented in clinical trials, and an inclusive research process may create lasting impact on furthering medical education and patient education.”
“Scalp psoriasis can be particularly burdensome to patients as it often impacts more visible areas of the body, including the hairline, forehead, neck, and around the ears, triggering feelings of self-consciousness that limit many people’s lifestyle choices," said Jennifer Davidson, DO, vice president of Medical Affairs, Immunology, Johnson & Johnson Innovative Medicine, in the news release. "The VISIBLE study suggests that people of color continue to experience undertreatment, with many participants not receiving a biologic option prior to this trial enrollment. The insights from the study aim to empower diverse patients with moderate to severe plaque and scalp psoriasis to learn more about their treatment options and initiate informed discussions with their providers."
In an exclusive interview with Dermatology Times, Jennifer Soung, MD, board-certified dermatologist and director of clinical research at Southern California Dermatology, provided in-depth insights into the VISIBLE study and its real-world implications for clinicians.
Q&A
Dermatology Times: The VISIBLE study focuses on patients with skin of color and highlights underrepresentation in clinical research. Can you please discuss how inclusive research processes may create lasting impacts on furthering medical education and patient education?
Jennifer Soung, MD: VISIBLE is an ongoing first-of-its-kind study 100% dedicated to patients with non-white skin or diverse skin tones with plaque and scalp psoriasis. The study design is unique in that it used a holistic, community-driven approach that leads with intentional site and investigator selection from diverse communities, involvement of a racially and ethnically diverse steering committee of dermatologists involved in protocol development and study execution, community engagement and awareness building, as well as educational and cultural training support for clinical investigators. It also used colorimetry as an objective method to determine baseline skin tone and to track progress of the discoloration of the skin when psoriasis lesions heal with darker or lighter patches.
The collective data from both cohort A and cohort B reinforces that the unique study design of VISIBLE is a successful model to enroll, retain, and support people with non-white skin or diverse skin tones who have historically been undertreated and underrepresented in clinical trials. Ultimately, we believe that the study design will generate key learnings for the medical community that we hope can be applied more broadly across healthcare to ensure that treatments work across all diverse populations and not just white skin.
Additionally, recognizing psoriasis can be more challenging in individuals with darker skin tones as it may appear purple, gray or darker brown rather than the typical reddening seen in lighter skin. This difference in presentation can sometimes lead to delayed or incorrect diagnoses for people with non-white skin or diverse skin tones. The study is expected to generate more than 20,000 images capturing disease presentation in diverse patients across all skin tones upon the study’s completion and will help providers and patients be better equipped to recognize these conditions on darker skin tones and treat them appropriately.
Dermatology Times: Scalp psoriasis is considered difficult to treat and often more severe in patients with skin of color. Can you discuss any specific challenges faced in treating scalp-predominant moderate-to-severe psoriasis and how guselkumab addresses these challenges?
Soung: Scalp psoriasis is a clinically proven difficult-to-treat area that some patients and doctors can mistake for other conditions, such as dandruff. It’s also believed that scalp psoriasis is more common and more severe in people with non-white skin or diverse skin tones. Further, because textured hair requires less washing, scalp psoriasis often advances to more severe stages in these patients and they often have a greater degree of scaling, making it harder to remove flakes.
Beyond the physical symptoms, scalp psoriasis can take a significant emotional toll. When left unmanaged, it can spread to the forehead and appear on areas of the body that are highly visible, which can make patients feel self-conscious and can even impact their lifestyle choices, down to the clothes that they choose to wear. The VISIBLE study found that people with non-white skin or diverse skin tones with scalp psoriasis remain undertreated, suggesting that the physical and emotional burden of the disease is significant.
Tremfya (guselkumab) is an FDA-approved biologic therapy, which can help to reduce the inflammation caused by psoriasis by targeting overactive cells in the immune system, disrupting the cycle of the disease and helping to improve symptoms. The data from the VISIBLE study demonstrates rapid and significant clearance in moderate to severe scalp psoriasis and significant improvement in scalp itch, as well as patient-reported health-related quality of life outcomes, including post-inflammatory pigmentation at 16 weeks across skin tones. To close the treatment gap that many people with non-white skin or diverse skin tones experience, the findings reinforce that it is critical for patients and providers to discuss advanced therapies like biologics when developing individualized treatment plans.
Dermatology Times: How can dermatologists integrate these recent findings into their practice for the benefit of their patients with scalp psoriasis?
Soung: The images and unique insights generated by the VISIBLE study can help clinicians improve the disease journey and health outcomes for people with non-white skin or diverse skin tones by equipping them with more information and visual tools that can be used when developing individualized treatment plans with patients.
The data also acts as a reminder on the importance of shared decision-making when treating patients with plaque and scalp psoriasis. A person’s culture and overall beliefs regarding their healthcare can shape how they engage with their doctor, so as physicians, it’s critical that we help to build trust, see and treat the full person, and make disease management decisions collaboratively to ensure our patients’ needs are being met.
References
- TREMFYA (guselkumab) demonstrates significant and rapid scalp psoriasis clearance in people of color in new large phase 3b study. News release. Johnson & Johnson. January 22, 2024. Accessed January 22, 2024. https://www.jnj.com/tremfya-guselkumab-demonstrates-significant-and-rapid-scalp-psoriasis-clearance-in-people-of-color-in-new-large-phase-3b-study
- Alexis A, McMichael A, Bhutani T, et al. VISIBLE cohort b: guselkumab demonstrates significant scalp clearance at week 16 in participants with moderate-to-severe scalp psoriasis across all skin tones. Presented at: Maui Derm Hawaii 2024; January 22-26, 2024; Maui, HI.
- Stein Gold L, Soung J, Heath CR, et al. VISIBLE cohort b: guselkumab improves key health-related quality of life measures at week 16 in participants with moderate-to-severe scalp psoriasis across all skin tones. Presented at: Maui Derm Hawaii 2024; January 22-26, 2024; Maui, HI.
- Yeung J, Vashi N, Shahriari M, et al. VISIBLE cohort b: guselkumab improves patient reported impact of skin discoloration at week 16 in participants with moderate-to-severe scalp psoriasis across all skin tones. Presented at: Maui Derm Hawaii 2024; January 22-26, 2024; Maui, HI.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.










