- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
IDP-126 Gel Demonstrates Superior Efficacy in Moderate-to-Severe Acne
Author(s):
Researchers said the treatment is both efficacious and safe in patients ages 9 and older.
IDP-126 gel demonstrated superior effectiveness in treating young patients with moderate-to-severe acne when compared with a vehicle-control treatment, according to a recent study.1
Pixel-Shot/AdobeStock
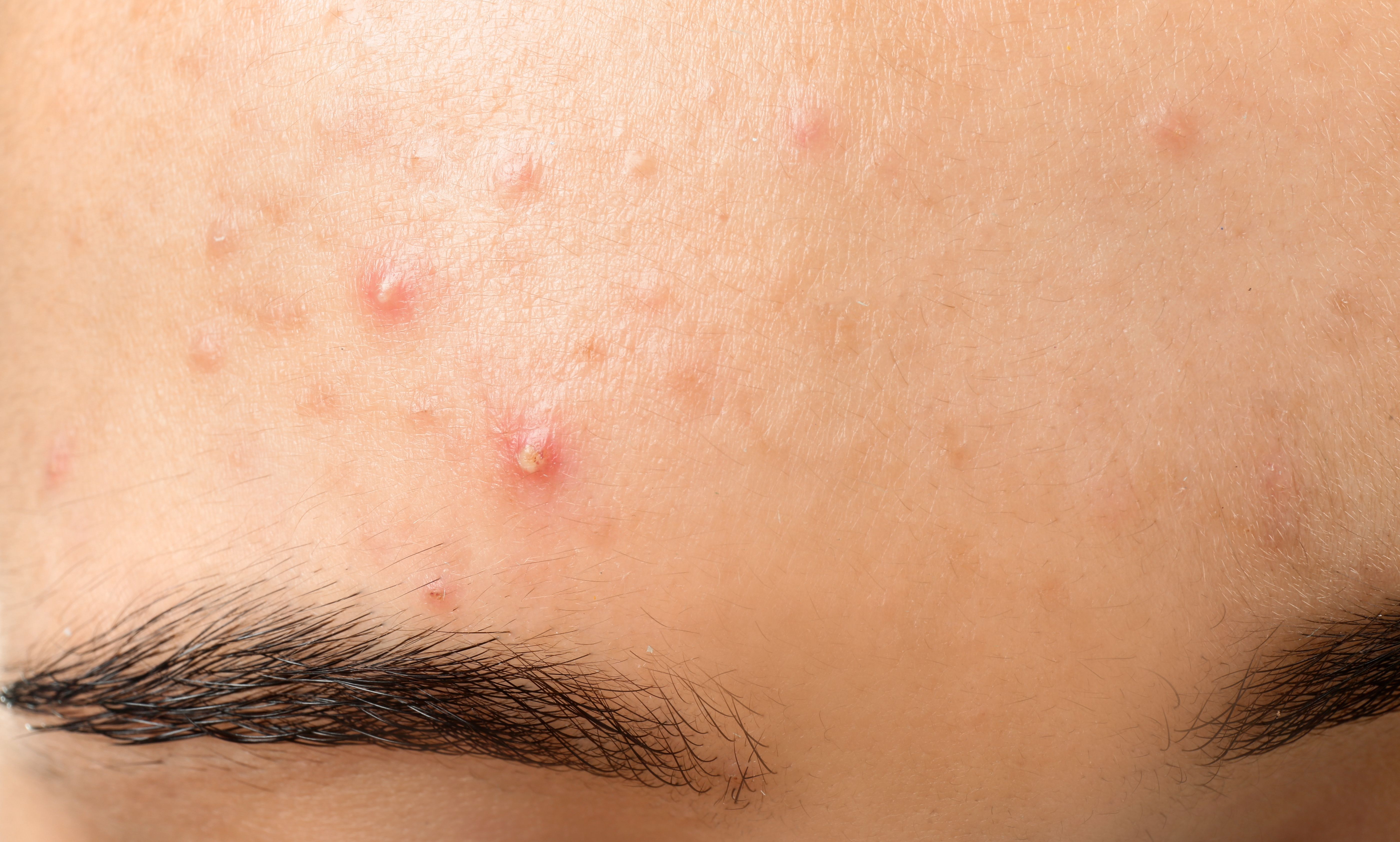
IDP-126, or topical clindamycin phosphate 1.2% benzoyl peroxide 3.1% adapalene 0.5% gel, is currently in development as the first fixed-dose triple-combination formula treatment for acne.
In order to establish the safety and effectiveness of IDP-126 gel in patients ages 9 and older, researchers conducted a phase 2 randomized, double-blind, parallel-group, placebo-controlled study across several treatment centers.
Participants (n=741) were at least 9 years of age and had been diagnosed with moderate-to-severe acne. Upon the start of the study, researchers randomized participants into 2 groups: an intent-to-treat (ITT) group comprised of pediatric patients (n=394), and a safety population (n=388).
From the ITT group, researchers further assigned participants into several treatments, including IDP-126 (n=76) and 3 dyad combination gels. These included benzoyl peroxide 3.1%/adapalene 0.15% (n=87); clindamycin phosphate 1.2%/benzoyl peroxide 3.1% (n=77); and clindamycin phosphate 1.2%/adapalene 0.15% (n=78). 76 participants were included in the vehicle-control group.
Participants applied their assigned treatment on a once-daily basis for a 12-week period.
Throughout the duration of the study, researchers gathered safety and efficacy data through several forms of assessments and measurements, including treatment success, inflammatory and non-inflammatory lesion counts, Acne-Specific Quality of Life (Acne-QoL), treatment-emergent adverse events (TEAEs), and cutaneous safety and tolerability.
Of all participants, those using IDP-126 gel achieved treatment success at a significantly higher rate than those assigned to other treatment groups. By the conclusion of the study, 55.8% of IDP-126 users had achieved success, whereas on average, 30.8% to 33.9% of participants in the ITT group utilizing dyad combination gels achieved success.
Additionally, participants in the IDP-126 group achieved greater success in reductions of inflammatory or non-inflammatory lesions from baseline than other participants, with significant reductions noted as early as 2 weeks into the study.
Regarding Acne-QoL scores, IDP-126 users experienced average improvements of 5.5 to 8.2 by week 12, whereas dyad combination gel users experienced improvements of 3.5 to 7.5, and members of the vehicle-control group improved by an average of 2.1 to 4.0.
Despite this, members of the clindamycin phosphate/adapalene treatment group had higher improvements to their role-emotional domain score when compared with the other treatment groups.
Members of the IDP-126 group more frequently experienced TEAEs. Reported adverse events were predominantly mild-to-moderate in nature and typically included pain or erythema at application site.
“Younger patients with acne experience considerable depreciation of quality of life and psychosocial function. IDP-126 treatment led to generally greater improvements in Acne-QoL domain scores versus dyad combinations or vehicle,” study authors wrote. “Pediatric participants experienced smaller improvements in all Acne-QoL domain scores with IDP-126 treatment than the overall study population; however, their mean baseline scores were higher, limiting the amount of improvement possible. Nevertheless, Acne-QoL improvements demonstrate the benefit of IDP-126 on pediatric patients' quality of life.”
Reference
- Eichenfield LF, Stein Gold L, Kircik LH, et al. Triple‐combination clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel for moderate‐to‐severe acne in children and adolescents: Randomized phase 2 study. Ped Dermatol. 2023. doi:10.1111/pde.15283
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











