- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
IDP-126 Gel Led to Improvements in Erythema Among Black Patients With Acne
Author(s):
A post hoc analysis evaluated the safety, efficacy, and tolerability of IDP-126 gel in Black patients with moderate to severe acne, of which there are limited data and literature describing its outcomes in this patient population.
Seventyfour/Adobe Stock
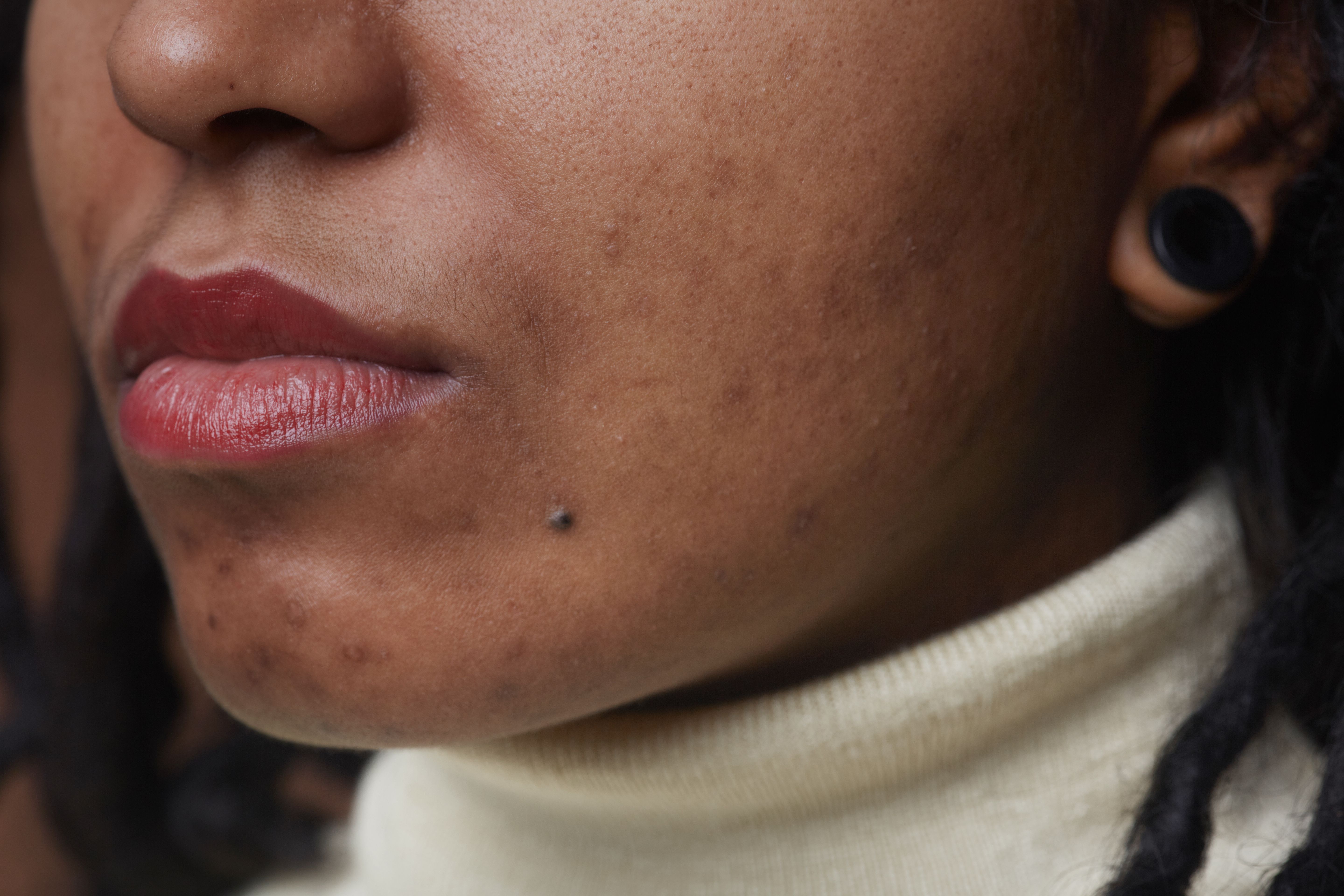
A fixed-dose of clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% (IDP-126) gel was safe, well-tolerated, and efficacious in reducing investigator-assessed erythema in Black patients with acne, according to a poster presented at the 2023 Society of Dermatology Physician Assistants Fall Conference in Nashville, TN.1
According to study authors Callender et al, literature and data describing patient outcomes for patients with skin of color in relation to this fixed-dose combination are limited. It is important, they noted, to be conscientious of minimizing skin irritation in patients with more melanin-rich skin due to risk of pigmentary alterations such as hyperpigmentation and hypopigmentation.
The pooled, post hoc analysis examined and analyzed the results of 2 identical phase 3 studies (NCT04214639; NCT04214652) involving patients ages 9 years and older with moderate to severe acne as defined by a score of 3 or 4 for on the Evaluator’s Global Severity Score scale.
Patients were randomized using a 2:1 ratio, wherein 2 patients were assigned to once-daily treatment with IDP-126 gel for every 1 patient assigned to treatment with a vehicle control gel. Both patient groups were provided with identical cleansers, lotions, and sunscreens on an as-needed basis.
Of 363 patients in the pooled intent-to-treat population, approximately 14.9% self-identified as Black.
As a result of treatment with IDP-126 gel, patients self-identifying as Black experienced improvements in erythema as assessed by investigators. Despite clinical improvements in erythema, investigators noted that no increases or incidences of hyperpigmentation or hyperpigmentation were evident. Rates of erythema had decreased by more than 10% from baseline to week 12 of treatment.
IDP-126 was deemed both safe and well-tolerated in this patient population, with only mild and moderate treatment-emergent adverse events (TEAE) reported among Black patients in the studies. The most commonly-reported TEAE was application site pain, followed by application site pruritus.
“Minimizing irritation is a key goal in managing acne in patients with skin of color, given the higher risk of pigmentary alterations in melanin-rich skin,” study authors wrote. “Despite the limited number of self-identified Black participants in these phase 3 studies, these post hoc analyses add valuable information to the limited literature describing treatment effects and tolerability of fixed-dose combination acne treatments in Black individuals.”
Reference
- Callender V, Cook-Bolden F, Kircik L, et al. Safety and tolerability of fixed-dose clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel in Black participants with acne. Poster presented at the 2023 Society of Dermatology Physician Assistants Fall Conference, October 26-29; Nashville, TN.
Make sure to keep up to date with the latest in coverage from the conference and subscribe to Dermatology Times to receive daily email updates.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.















