- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
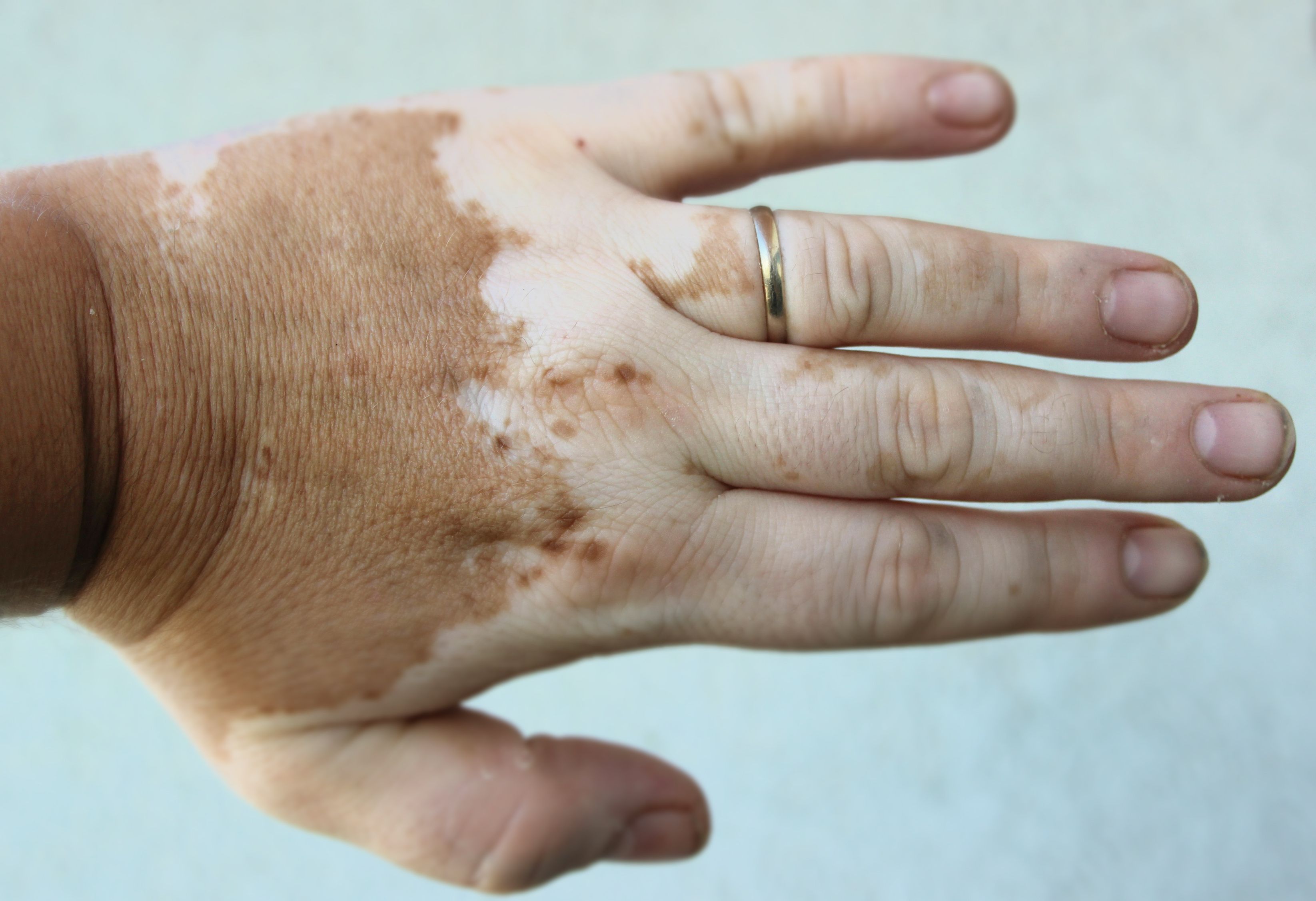
Incyte Announces Positive 52-Week Data at EADV of Povorcitinib for Extensive Nonsegmental Vitiligo
Author(s):
Longer-term use of povorcitinib demonstrated further improvement in total body and facial repigmentation.
Incyte recently announced positive 52-week data from a phase 2b clinical trial evaluating the safety and efficacy of povorcitinib (INCB54707), an investigational oral JAK1 inhibitor, for the treatment of extensive nonsegmental vitiligo in adult patients. Findings were presented at the European Academy of Dermatology and Venereology (EADV) Congress 2023 in Berlin, Germany. The results build upon previously announced data and suggest significant advancements in potential treatment.1
Using the Total Vitiligo Area Scoring Index (T-VASI) for total body depigmentation and the facial Vitiligo Area Scoring Index (F-VASI) for facial depigmentation to measure depigmentation improvements, study results showed:
- Total body depigmentation improvement: The mean percentage improvement in total body depigmentation from baseline at week 52 was substantial across all treatment groups. Povorcitinib doses of 15 to 75 mg, 45 mg, and 75 mg exhibited significant improvements of 40.7%, 42.7%, and 41.3%, respectively, compared to 18.1% for the placebo-to-75 mg group.
- Facial depigmentation improvement: Facial depigmentation saw notable improvements at week 52, with percentages of 63.6%, 63.8%, and 64.4% for the 15 to 75 mg, 45 mg, and 75 mg povorcitinib groups, respectively. The placebo-to-75 mg group showed a 54.8% improvement.
Secondary end points included:
- More patients in the povorcitinib groups achieved a ≥50% reduction from baseline in T-VASI at week 52 compared to week 24, with the highest percentage in the 15 to 75 mg group at 45.2%.
- For facial depigmentation results, more patients achieved a ≥50% and >75% reduction from baseline in F-VASI at week 52 compared to week 24, especially in the 45 mg group.
- Povorcitinib was well-tolerated at all doses, with the most common treatment-emergent adverse events including COVID-19, increased blood creatine phosphokinase, acne, fatigue, and headache.
- The durability of response following treatment discontinuation was observed in a small sample size, suggesting a lasting impact on repigmentation.
Incyte’s phase 2b study (NCT04818346) was a randomized, double-blind, placebo-controlled study enrolling 171 adult patients with extensive nonsegmental vitiligo. The primary endpoint was the percentage change from baseline in T-VASI at week 24, and key secondary endpoints included the percentage of patients achieving ≥50% reduction from baseline in the T-VASI T-VASI50 at week 24.
“These 52-week results further support earlier data and reinforce the efficacy profile and potential of povorcitinib as an oral treatment for patients with extensive nonsegmental vitiligo,” said Kurt Brown, MD, vice president and povorcitinib global program head at Incyte, in the news release. “At Incyte, we are deeply committed to addressing unmet needs in the vitiligo community and understanding how this disease can affect patients’ lives. Today’s data highlight exciting progress as we work to bring new potential treatment options to patients living with this immune-mediated skin condition.”
Reference
- Incyte announces positive 52-week data from phase 2b study evaluating povorcitinib (INCB54707) in patients with extensive nonsegmental vitiligo. Incyte. News release. October 11, 2023. Accessed October 11, 2023. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-positive-52-week-data-phase-2b-study-evaluating
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.