- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
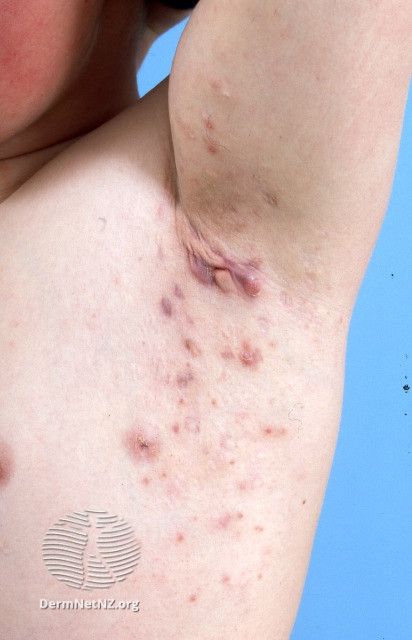
Izokibep Fails in Phase 2b/3 Hidradenitis Suppurativa Trial
Author(s):
The IL-17A inhibitor did not meet its phase 2b/3 clinical trial primary end point.
Acelyrin recently announced that its investigational IL-17A inhibitor, izokibep, failed to meet the primary end point of its phase 2b/3(NCT05355805) clinical trial for the treatment of hidradenitis suppurativa (HS).1
Thirty-nine percent of patients treated with 160 mg of izokibep once a week demonstrated at least a 75% decrease in HS clinical response score (HiSCR75) score at 16 weeks. Additionally, 29% of patients treated with placebo achieved a similar HiSCR75 score. These results did not prove a significant treatment effect, with a P value of 0.3278.
Only 34% of patients who received izokibep twice a week achieved HiSCR75 at 16 weeks which was not statistically different enough from the placebo group.
In part B of Acelyrin’s phase 2b/3 clinical trial, 175 patients with moderate-to-severe HS were enrolled and given 160 mg doses either once- or twice-weekly. The primary end point of the trial was HiSCR75 calculated by a non-responder imputation (NRI) method, “which according to Acelyrin was negatively affected in part B by high rates of discontinuation as early as 4 weeks into the trial.” According to Acelyrin, there was also an unexpected increase in placebo response which led to izokibep’s non-significant treatment effect.
To account for the discontinuations, Acelyrin used the last observation carried forward (LOCF) sensitivity method, which found that the once-weekly dosing schedule of izokibep was significantly better than placebo at inducing HiSCR75 at week 16. A modified NRI analysis also found that izokibep elicited higher rates of HiSCR75 than placebo with strong statistical significance. Even though izokibep did not show statistical significance in either NRI or LOCF analyses, Acelyrin detected stronger efficacy signals related to HiSCR100, with a 100% decrease in scores from baseline.
“The consistent and early achievement of HiSCR100, along with our prior izokibep experience in Psoriatic Arthritis, continues to demonstrate the potential of izokibep for resolution of disease, especially in difficult to treat tissues,” said Acelyrin’s CEO Shao-Lee Lin in the news release.
Izokibep’s safety profile was consistent with previous reports. In total, 31 patients dropped out of the trial, which were mostly unrelated to adverse events.
Currently, Acelyrin still has an ongoing phase 3 trial investigating izokibep for HS (NCT05905783).
Reference
- Manalac T. Acelyrin’s lead assetizokibep fails in late-stage hidradenitis suppurativa trial. BioSpace. News release. September 12, 2023. Accessed September 12, 2023. https://www.biospace.com/article/acelyrin-s-lead-asset-izokibep-fails-in-late-stage-hidradenitis-suppurativa-trial/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.