- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
J-Code for Ycanth Officially Launches Today
Author(s):
The permanent J-code for the molluscum contagiosum treatment is now live.
Verrica Pharmaceuticals’ VP-102 (Ycanth) was approved by the US Food and Drug Administration (FDA) for the treatment of molluscum contagiosum on July 21, 2023. Image courtesy of Verrica Pharmaceuticals.
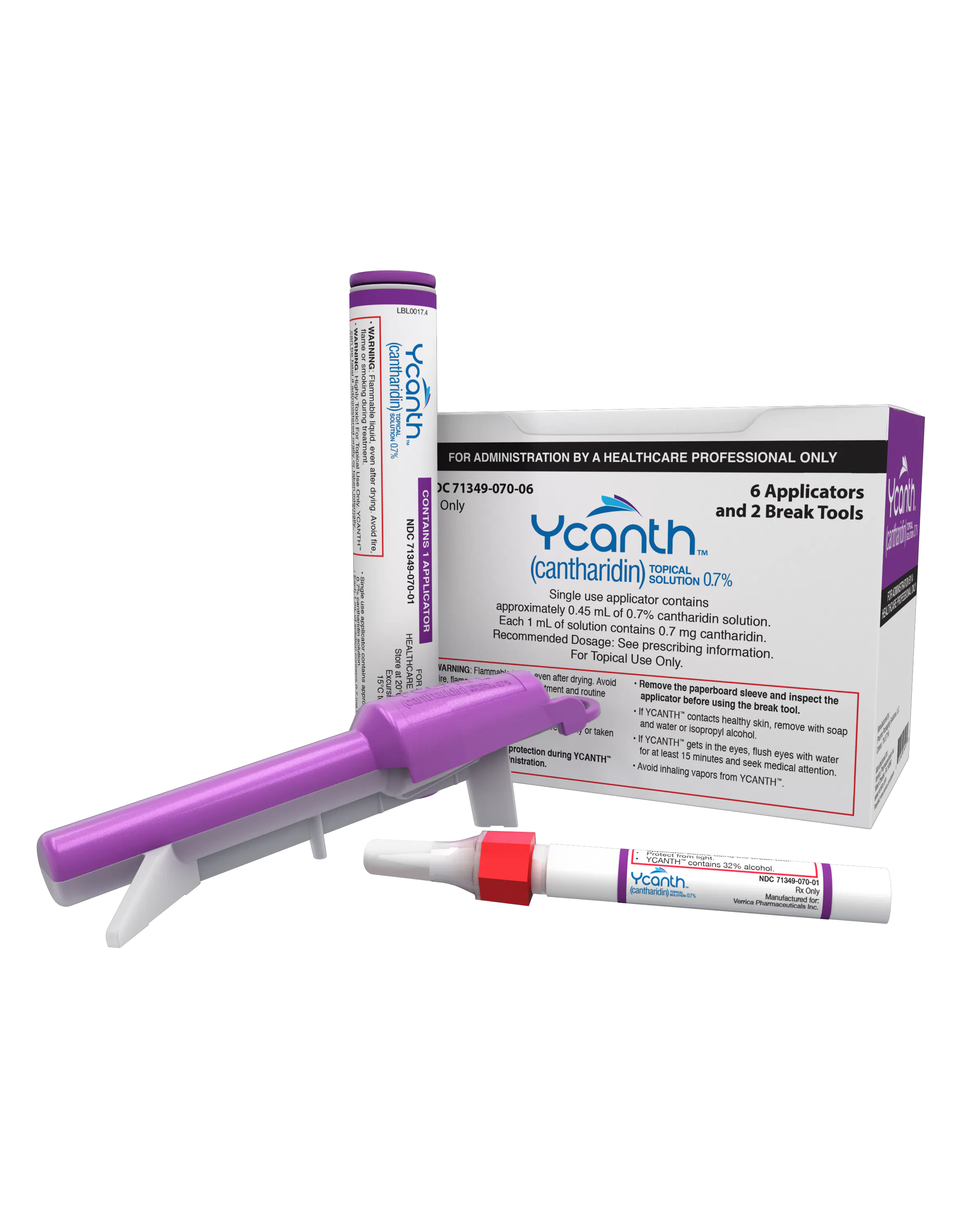
The US Centers for Medicare & Medicaid Services (CMS) has officially launched a permanent and product-specific J-code, J7354, for cantharidin topical solution 0.7% (Ycanth), the first FDA-approved treatment for molluscum contagiosum. The J-code announcement came in January and was fully published today.1
“By securing a permanent J-Code for YCANTH, we have successfully reached a critical milestone in our commercial strategy that we expect will help us accelerate YCANTH utilization among the U.S. Medicaid and Medicare patient populations,” said Ted White, President and Chief Executive Officer of Verrica Pharmaceuticals, previously said in a news release. “In addition to greater patient access, we also anticipate a permanent J-Code will result in a more streamlined billing and reimbursement process for YCANTH.”
Ycanth was approved by the FDA for the treatment of molluscum contagiosum in adults and pediatric pages aged 2 years and older on July 21, 2023. The approval was based on positive data from 2 identical phase 3 randomized, double-blind, multicenter clinical trials (CAMP-1 and CAMP-2). The exclusive drug-device combination solution features a carefully regulated formulation of cantharidin (0.7% w/v) administered through a disposable applicator.2 Ycanth was launched commercially in September 2023. Molluscum contagiosum accounts for approximately 1% of all diagnosed skin conditions and is 1 of the 50 most common skin conditions. Molluscum contagiosum most commonly affects pediatric patients, beginning around age 6 years.
"Until now, we finally have our very first FDA-approved product, Ycanth, which was known as VP-102, which is a drug-device combination product that applies a very small, precise amount of cantharidin and a surgical dye and is applied in office to the patient’s lesions," said Shanna Miranti, MPAS, PA-C in a prior interview with Dermatology Times. "We can very specifically treat the individual raised bumps. There is a purple-stained dye that is contained in the medication combination, as well, that allows us to see the precise application location, so we know exactly what bumps have been treated and what bumps have not. This will truly start to help those providers who see molluscum—and there are a lot of us out there—gain consistency in our treatments."
Before this approval, there were no specific guidelines to treat the condition and many dermatology clinicians were using compounded cantharidin. However, not all compounds are created equal and there is a lot of variability. Miranti went on to explain in a previous interview, "In early June 2023, the FDA came out with a pretty strongly worded consumer warning against using compounded cantharidin. Because of the lack of consistency across manufacturers, both in the country or manufacturers in Canada, the FDA was warning providers and patients that there was no FDA-approved option yet. The FDA warned that patients needed to be very careful and cautious about products that were commercially available on Amazon or over the counter or through mail-order websites that have not actually proven efficacy to the FDA. There are a number of potential concerns and potential adverse effects that can occur from products that are not FDA approved. Because of the FDA’s strong warning, we as providers really need to pay attention to that. We need to start utilizing the options that are truly FDA approved and have been proven both safe and efficacious for our patients."
As solutions for mollescum contagiosum roll out, Miranti addressed a couple of small growing pains and said, "There will be either a buy-and-bill system or there is an Rx model as well, where we can write a patient a prescription and then have the product sent to our office and have the treatment ready for that patient’s next appointment. We will be able to devise a system that works for our procedures and our policies in the office and hopefully will help optimize workflow. This is a product that’s going to be ready to use and ready to go when our patient is there seeking treatment."
Have you utilized the latest treatment options for molluscum contagiosum? What do you think of the current treatment landscape available? We would love to hear from you. Email us at DTEditor@mmhgroup.com.
References
- Verrica Pharmaceuticals Receives Permanent J-Code (J7354) for YCANTH from Centers for Medicare and Medicaid Services. GlobeNewswire. January 29, 2024. Accessed January 29, 2024. https://www.globenewswire.com/news-release/2024/01/29/2818804/0/en/Verrica-Pharmaceuticals-Receives-Permanent-J-Code-J7354-for-YCANTH-from-Centers-for-Medicare-and-Medicaid-Services.html
- Verrica Pharmaceuticals Announces FDA Approval of YCANTH (cantharidin) topical solution 0.7%. Verrica Pharmaceuticals. July 21, 2023. Accessed January 29, 2024. https://verrica.com/press_release/verrica-pharmaceuticals-announces-fda-approval-of-ycanth-cantharidin-topical-solution-0-7/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














