- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
JAK inhibitors help address limitations of biologics
Author(s):
As a class, JAK inhibitors look like the best oral targeted therapeutic option when topical cortical steroids and/or topical calcineurin inhibitors plus emollients fail.
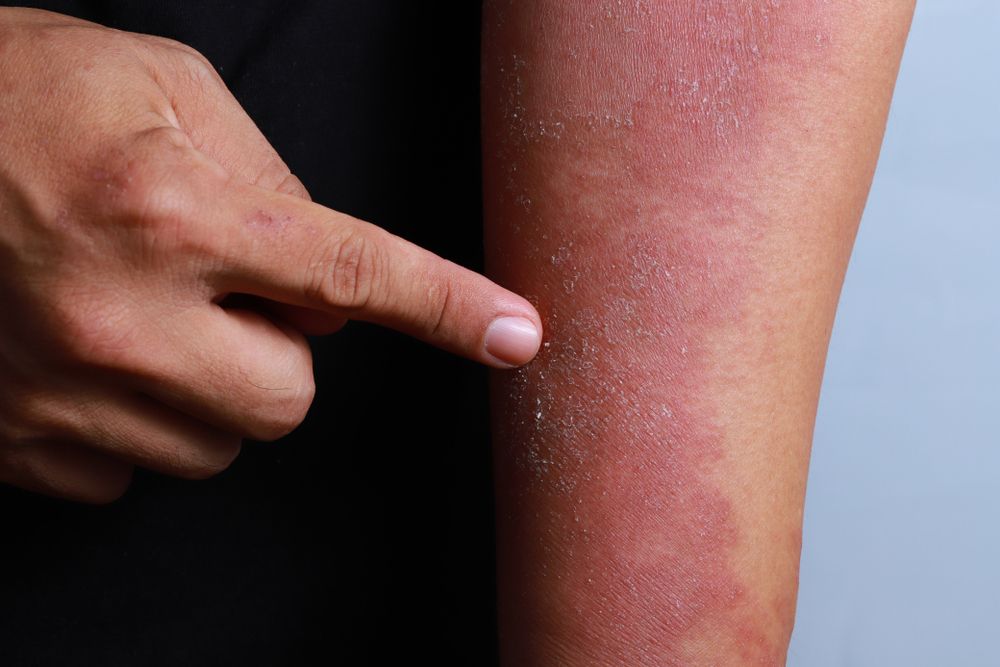
As with psoriasis, there is a growing movement towards using targeted therapies to improve outcomes and minimize side effects in atopic dermatitis.
“JAK inhibitors are an important focus of therapeutic research,” says Maria Alexandra Rodrigues, department of dermatology, Centro Hospitalar e Universitário do Porto, in Portugal, who recently co-authored a review of the evidence of efficacy and safety for JAK inhibitors for the treatment of the condition in Journal of Dermatological Treatment.1 Several JAK inhibitors are in phase 2 and 3 clinical trials as oral therapies for moderate-to-severe atopic dermatitis or as topical treatments for mild-to-moderate disease.
“Results thus far are encouraging, with the majority of the patients achieving the primary outcome of their trial as well as a favorable safety profile,” she says. “JAK inhibitors will most certainly be the first oral targeted option when topical therapy fails. With good oral bioavailability and lack of immunogenicity, they address some of the limitations of biologics.”
It is yet to be determined whether selective JAK 1 inhibitors or non-selective JAK inhibitors will provide the best equilibrium of efficacy versus side effects, she adds. “Although, an unmet need exists in the topical treatment of atopic dermatitis, the position in the therapeutic ladder for topical JAK inhibitors is less clear.”2
In atopic dermatitis, there are multiple upregulation of cytokine families, and JAK inhibitors can block a range of cytokines, growth factor and/or hormone receptor signaling pathways which make them an important candidate treatment.
The first published randomized clinical trial demonstrating a clinical benefit of a topical JAK inhibitor in atopic appeared in 2016, and within 2 years, seven different agents were undergoing randomized trials targeting the pathway, and so far, the data suggest a benefit.
Both selective JAK 1 inhibitors upadacitinib (ABT-494, Abbvie) and PF-04965842 (abrocitinib, Pfizer) have received breakthrough therapy designation from the U.S. Food and Drug Administration for treatment of patients with moderate-to-severe AD. Preliminary phase 2 trial data suggest upadacitinib is associated with even better outcomes than dupilumab (Dupixent, Sanofi) with improvement in pruritus as soon as week one and skin improvement as soon as week two.
However, Rodrigues points out that it has yet to be determined whether the short time to response is a characteristic feature of upadacitinib, of JAK 1 selective inhibitors or of JAK inhibitors in general. Baricitinib (Olumiant, Eli Lilly), a non-selective JAK inhibitor, also reported improvement in pruritus as soon as week one.3
As a class, JAK inhibitors look like the best oral targeted therapeutic option when topical cortical steroids and/or topical calcineurin inhibitors plus emollients fail, she says.
“Upadacitinib data demonstrate that selective JAK 1 inhibition is sufficient for high efficacy results and time will show if there is also a benefit in the reduction of serious adverse effects like tuberculosis and herpes zoster,” she says.
The most extensive safety data for JAK inhibitors has come from the use of tofacitinib (Xeljanz, Pfizer), ruxolitinib and baricitinib in rheumatoid arthritis and myelofibrosis. In these conditions use of JAK inhibitors has been associated with a slight increased risk of tuberculosis and herpes zoster, but the most frequent adverse effects were nasopharyngitis and upper respiratory tract infections. There was also a slight increase in creatine phosphokinase and changes in hemogram.
“Safety data in patients with atopic dermatitis is still limited as the majority of phase 2 trials are ongoing or awaiting peer reviewed publication but to date there are no tuberculosis, herpes zoster or increased malignancy risk cases reported,” Rodrigues says.
Complete blood count, creatinine and liver transaminases should be assessed at baseline, along with CPK, which should be monitored during therapy and a close eye kept for signs of infection.
“Although less data exist at this time regarding the safety of topical JAK inhibitors, it is unlikely that these agents will require any laboratory monitoring,” she says.
References:
Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat. 2019;:1-8.
2 Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe atopic dermatitis: a phase II, multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol. 2018;178(2):424-432.
3 Guttman-yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913-921.e9.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











